Abstract
Purpose
Obstructive sleep apnea (OSA) is a widespread comorbidity of obesity. Nasal continuous positive airway pressure (CPAP) has been demonstrated very effective in treating patients with OSA. The aims of this study were to investigate whether or not cardiopulmonary exercise testing (CPET) can characterize patients with OSA and to evaluate the effect of nasal CPAP therapy.
Methods
An observational study was conducted on patients with moderate to severe obesity and suspected OSA. All patients underwent cardiorespiratory sleep study, spirometry, and functional evaluation with ECG-monitored, incremental, maximal CPET.
Results
Of the 147 patients, 94 presented with an apnea–hypopnea index (AHI) ≥ 15 events/h and were thus considered to have OSA (52 receiving nasal CPAP treatment; 42 untreated) while 53 formed a control group (AHI < 15 events/h). Patients with untreated OSA showed significantly lower oxygen uptake (VO2), heart rate, minute ventilation (VE), and end tidal carbon dioxide (PETCO2) at peak exercise compared to controls. Patients receiving nasal CPAP showed higher VE and VO2 at peak exercise compared to untreated patients. A difference in PETCO2 between the maximum value reached during test and peak exercise (ΔPETCO2 max-peak) of 1.71 mmHg was identified as a predictor of OSA.
Conclusion
Patients with moderate to severe obesity and untreated OSA presented a distinctive CPET-pattern characterized by lower aerobic and exercise capacity, higher PETCO2 at peak exercise associated with a lower ventilatory response. Nasal CPAP treatment was shown to positively affect these cardiorespiratory adaptations during exercise. ΔPETCO2 max-peak may be used to suggest OSA in patients with obesity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a relatively common condition in the adult population, closely associated with obesity [1]. OSA is characterized by repeated episodes of apnea and hypopnea during sleep resulting in intermittent hypoxia and hypercapnia, frequent arousals with sleep fragmentation, and daytime sleepiness. It also leads to secondary sympathetic activation, oxidative stress, systemic inflammation, and increased cardiovascular risk [2].
Cardiopulmonary exercise testing (CPET) is highly recommended for patients with increased cardiovascular risk and chronic diseases, like obesity. Indeed, different CPET parameters have been proposed as important prognostic markers, while others are used for diagnostic testing and cardiopulmonary screening. Despite an increasing scientific interest in OSA in recent years [3], very few data investigating the effect of OSA on cardiopulmonary function in patients with severe obesity are currently available [4, 5]. Most studies have shown reduced maximal aerobic exercise capacity in patients with OSA compared to control subjects [6, 7]. Previous studies showed improvements in cardiac function and cardiorespiratory fitness in patients with OSA after eight weeks of nasal continuous positive airway pressure (CPAP) treatment [8, 9]. Indeed, nasal CPAP is the gold-standard treatment for moderate to severe OSA and has been proven to reduce cardiovascular mortality and non-fatal cardiovascular events in this population [10].
The present study aimed, first to characterize the cardiopulmonary function of patients with moderate to severe obesity affected by OSA, investigating a possible distinctive cardiopulmonary response to exercise. Second, we aimed to evaluate if CPAP therapy may affect cardiorespiratory efficiency and ventilatory drive during exercise.
Material and methods
Participants and protocol
In this observational cross-sectional study, we evaluated 185 patients affected by moderate to severe obesity and suspected OSA, consecutively recruited within the Veneto Region diagnostic-therapeutic pathway for patients with obesity. These patients underwent cardiorespiratory sleep study analysis at University Hospital of Padova from February 2014 to January 2020 for suspicion of OSA based on clinical evaluation and a validated questionnaire (Epworth Sleepiness Scale). During the same period, these patients had been referred for functional evaluation to the Sport and Exercise Medicine Division. This study was performed in accordance with the Declaration of Helsinki and approved by the local ethics committee (99n/AO/21); all participants provided written informed consent. Inclusion criteria were age between 18 and 70 years, body mass index (BMI) > 35 kg/m2, and suspected OSA. Exclusion criteria were significant heart, lung, or musculoskeletal disease that would impede maximal exercise testing. A subgroup of patients affected by OSA was treated with CPAP for at least 8 weeks before functional evaluation. The remaining patients with OSA were not receiving CPAP due to intolerance or were waiting to start therapy. Thus, the patients were divided in three groups:
-
Patients affected by obesity (Ob)
-
Patients affected by obesity and OSA (Ob-OSA)
-
Patients affected by obesity and OSA treated with CPAP (Ob-CPAP)
Cardiorespiratory sleep study
The cardiorespiratory sleep study was performed using the SOMNOtouchTM® NIBP device (SOMNOmedics Italia), for at least 8 h per night. Peripheral oxygen saturation, respiratory flow in the upper airways, respiratory movements of the chest and abdomen, snoring phases, sleeping position, blood pressure levels, and ECG were recorded. These data were automatically analyzed by the DOMINO software® and subsequently validated by an expert operator according to the most recent American Academy of Sleep Medicine Reviewer criteria [11]. The polysomnographic data encompassed Apnea Hypopnea Index (AHI), minimum percentage oxyhemoglobin saturation (mSaO2%), mean percentage oxyhemoglobin saturation (meanSaO2%), percentage of time with oxyhemoglobin saturation percentage less than 90 (TS < 90%), and number of desaturations with oxyhemoglobin saturation < 90% (nSaO2 < 90%). We were unable to detect peripheral oxygen saturation of 13 participants. In this study, OSA was considered to be present in patients with an AHI of 15 or more events/h [11].
Cardiopulmonary exercise testing
Each patient was subsequently evaluated with incremental, maximal, ECG-monitored CPET (Jaeger Masterscreen-CPX, Carefusion). All tests were performed on treadmill (T170 DE, Cosmed), using the modified Bruce protocol. Criteria of exhaustion were a Borg rating of perceived exertion ≥ 18/20 associated with a Respiratory Exchange Ratio (RER) > 1.10, and/or a peak heart rate (HR) ≥ 85% of predicted HR max, and/or the achievement of a plateau of oxygen uptake (VO2). Arterial blood pressure and peripheral oxygen saturation were continuously monitored. Ventilatory and gas exchange measurements were sampled breath-by-breath to assess: VO2, minute ventilation (VE), ventilatory equivalents for carbon dioxide (VE/VCO2) and end-tidal pressures for oxygen and carbon dioxide (PETO2 and PETCO2). VE, VO2 and PETCO2 were determined also at the anaerobic threshold (AT) and respiratory compensation point (RCP) [12]. Differences between two PETCO2 values were presented as ΔPETCO2, i.e., the difference between the maximum PETCO2 value reached during testing and PETCO2 at peak exercise was defined as ΔPETCO2 max-peak.
Statistical analysis
Statistical analyses were performed with Statistical Package for Social Science (SPSS Inc., Chicago). Continuous variables are expressed as mean ± standard deviation and comparison between subgroups was performed with the one-way analysis of variance test with Bonferroni correction. Categorical variables were compared between groups using Pearson’s chi squared test. The relationship between continuous variables was evaluated by Spearman’s correlation coefficient (r). Receiver operating characteristic (ROC) curve was constructed to identify a parameter that can be used to discriminate Ob-OSA from Ob, maximizing sensitivity and specificity values. All reported probability values were two-tailed and a value of p < 0.05 was considered statistically significant.
Results
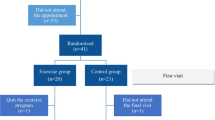
Of 147 patients with moderate to severe obesity eligible for study inclusion, 94 patients with OSA (52 Ob-CPAP and 42 Ob-OSA) were compared with 53 patients without OSA (Ob; Fig. 1). Demographic and anthropometric characteristics, lung function, and cardiorespiratory sleep study measurements of patients are shown in Table 1. No differences in age, gender, BMI, or sedentary lifestyle were found between groups. Major co-morbidities were dyslipidemia (68%), arterial hypertension (52%), and diabetes mellitus (23%); 30 patients were active smokers (20%).
Resting and exercise CPET parameters are presented in Table 2. Ob-OSA presented lower VO2 peak/kg when compared to Ob and Ob-CPAP (p = 0.014 and p = 0.019, respectively). Indeed, not considering patients in CPAP treatment, a significant, although weak, inverse correlation between VO2 peak/kg and AHI was identified (r = − 0.242, p = 0.018).
Evaluating patients’ cardiovascular response to exercise, data show that Ob-OSA presented increased diastolic blood pressure (DBP) at peak exercise compared to Ob-CPAP and Ob (p = 0.015 and p = 0.003, respectively), while no significant difference between groups on systolic blood pressure (SBP) at rest and at peak exercise was observed. Furthermore, maximal HR and HR reserve were higher in Ob than in Ob-OSA (both p = 0.003).
Finally, regarding patients’ respiratory gas exchange, Ob-OSA presented lower maximal VE compared to Ob-CPAP (p = 0.002) and higher PETCO2 at peak exercise (PETCO2 peak) compared to Ob (p = 0.004; Table 3). Indeed, not considering patients in CPAP treatment, PETCO2 peak showed a statistically significant positive correlation with AHI (r = 0.401, p < 0.001; Fig. 2). Moreover, data revealed a lower ΔPETCO2 max-peak when Ob-OSA was compared with Ob-CPAP and Ob (both p < 0.001; Table 3 and Fig. 3). ROC analysis demonstrated that ΔPETCO2 max-peak, used as predictor of Ob-OSA, showed an area under the curve of 0.811. A cut-off value of 1.71 mmHg can be proposed with a sensitivity of 81% and a specificity of 67% (Fig. 4).
Relationship between PETCO2 peak and AHI. Figure 2 shows the positive correlation between end tidal carbon dioxide at peak exercise (PETCO2 peak) and apnea–hypopnea index (AHI)
Response of PETCO2 during incremental exercise. Figure 3 shows the response of end tidal carbon dioxide (PETCO2) during incremental cardiopulmonary exercise testing in Ob (blue), Ob-CPAP (red), and Ob-OSA (green). Although PETCO2 max is similar among the three groups, it diverges at peak exercise (graphic above). In the graphic, it is possible notice that these differences in PETCO2 peak occur after RCP is achieved. REST, at rest; AT, anaerobic threshold; RCP, respiratory compensation point; PEAK, at peak exercise; MAX, maximum value reached during exercise testing
ROC curve analysis of ΔPETCO2 max-peak. Figure 4 shows receiver operating characteristic curve analysis of the difference in end tidal carbon dioxide pressure between the maximum value reached during exercise and peak exercise (ΔPETCO2 max-peak), as predictor of OSA. The red circle indicates the value of 1.71 mmHg, having a sensitivity of 81% and a specificity of 67%. AUC, area under the curve; CI 95%, 95% confidence interval
Discussion
This is the largest single study reporting cardiopulmonary exercise parameters in patients with OSA and moderate to severe obesity. To date, CPET has not been considered a useful tool in the evaluation of patients with suspected OSA, although the literature has already described distinguishing features that might be useful in the diagnostic process as well as during follow-up. This work aims to provide further evidence for the potential utility of CPET in the clinical management of these patients. The main results of our study are the following:
-
1.
Patients with moderate to severe obesity and OSA showed reduced aerobic capacity and exercise tolerance compared to patients with moderate to severe obesity without OSA.
-
2.
Although patients with OSA showed higher PETCO2 at peak exercise, a reduced ventilatory drive was observed.
-
3.
ΔPETCO2 max-peak may be proposed as a marker in CPET for patients with obesity and suspected OSA.
-
4.
Patients with OSA receiving CPAP therapy showed a cardiorespiratory response to exercise similar to controls without OSA.
Cardiorespiratory fitness
According to our data, Ob-OSA presented with lower cardiorespiratory fitness compared to Ob and Ob-CPAP. Different previous studies have evaluated exercise capacity in patients with OSA but only two of them analyzed patients with severe obesity [4, 5]. A recent systematic review and meta-analysis showed a reduced maximal aerobic capacity in patients with OSA, confirming its significant impact on patients’ cardiorespiratory fitness [3]. The reasons for these exercise limitations are not fully understood but different explanations have been proposed, suggesting a multifactorial impairment.
Patients with OSA present more frequently left and right ventricular diastolic dysfunction, respiratory alterations, restrictive pulmonary disease, and pulmonary hypertension, which may all contribute to a reduced maximal aerobic capacity [13, 14]. For this reason, patients affected by these diseases were excluded from this study. Indeed, lung function tests, breathing reserve, oxygen pulse at peak, and the absence of peripheral desaturation during exercise with normal VE/VCO2 slope were not consistent with major pulmonary or cardiac limitations to exercise.
Furthermore, a decreased maximal lactate concentration and its delayed elimination has been observed in patients with OSA during exercise when compared to age and BMI matched controls and this may indicate impaired glycolytic metabolism and reduced exercise tolerance [15].
Also, musculoskeletal damage has been proposed as possible cause of exercise impairment. Indeed, muscular biopsies have demonstrated structural and bio-energetic changes in skeletal muscle fibers, probably due to continuous or intermittent hypoxia [16].
Sleep-related hypopnea leads to excessive daytime sleepiness, which may affect the ability to achieve maximum exercise workload. Sleep deprivation has already been identified as a limiting factor in exercise time because it seems to increase perceived maximal effort [17]. Moreover, aerobic capacity is influenced by physical activity level, which is known to be lower in patients with OSA [18, 19].
Ob-CPAP exhibited higher aerobic capacity than Ob-OSA, comparable with controls. The continuous CPAP treatment effect on aerobic capacity in patients with OSA has been already described [2]. In fact, CPAP therapy for at least eight consecutive weeks was predominantly associated with significant improvements in VO2 max [8, 9, 20, 21]. CPAP therapy may normalize gas exchanges during sleep and contribute to structural and bio-energetic changes in skeletal muscles. Furthermore, CPAP associated reduction of sleep deprivation and better daytime alertness may help to increase motivation and performance during CPET, consequently leading to higher maximal aerobic capacity. Indeed, though CPAP therapy does not seem to improve quality of life scores, it has been shown to increase physical domains and vitality of patients with OSA patients [22].
Cardiovascular response
The reduced aerobic capacity found in Ob-OSA may at least in part be due to a lower HR response during exercise [4, 23]. Indeed, several other studies have reported a chronotropic impairment at peak exercise in patients with OSA suggesting a downregulation of beta-adrenergic receptors consequent to sympathetic hyperactivity [24].
The cardiovascular response to exercise was also characterized by a higher DBP at peak exercise in Ob-OSA, which is in line with results of previous studies [15, 25]. However, the Ob-CPAP group showed a similar DBP response during exercise compared to Ob. Indeed, CPAP therapy has been associated with a decrease in sympathetic hyperactivity as assessed by HR variability [20]. Excessive sympathetic activity may contribute to limit maximal aerobic capacity through peripheral vasoconstriction probably due to the activation of excitatory chemoreflex afferents.
Ventilatory response and gas-exchange
OSA is characterized by recurrent upper airway collapse during sleep which may cause CO2 retention leading to the onset of respiratory acidosis, resulting in compensatory renal retention of bicarbonate ions. This condition leads to a subsequent reduced respiratory frequency and daily ventilation. Although resting ventilation was similar between the three study groups, data showed a reduced ventilatory response to exercise in Ob-OSA. The continuous stimulus by chronic CO2 retention might cause a dysregulation of the metabolic set point that affects the ventilatory drive, causing it to be less sensitive to CO2 levels [26]. Moreover, a reduced ventilatory response at high exercise intensities may reduce patients’ exercise tolerance due to limited metabolic buffering and directly influence their maximal aerobic capacity. Indeed, the blunted respiratory drive cannot compensate for the increased respiratory demand during exercise and thus patients with OSA cannot eliminate the extra amount of CO2 produced during exercise, causing increased levels of PETCO2 at elevated intensities [27].
PETCO2 presents a specific pattern during exercise, which is mainly influenced by progressive accumulation of lactic acid during incremental exercise, the subsequent metabolic buffering, and the associated ventilatory response. PETCO2 rises until reaching the AT, remaining constant during isocapnic buffering until the RCP is reached. Afterwards, a further ventilatory surge exceeds the increase in the respiratory elimination of CO2 causing a physiological reduction in PETCO2. The trend of PETCO2 during the exercise phases seems similar in the different study groups until reaching the RCP, when Ob-OSA showed a lower decreasing trend in PETCO2 up to peak exercise. Indeed, 16 patients presented a PETCO2 peak higher than PETCO2 at the RCP and 15 of them belonged to Ob-OSA group. This is further supported by the positive correlation between PETCO2 peak and the severity of OSA. Despite the role of PETCO2 has already been evaluated during sleep in patients with OSA [28], there are only few published data investigating its behavior during exercise, and no information is currently available during CPET in patients with moderate-severe obesity. Our study outcomes in this specific population are in line with preceding studies showing higher PETCO2 at peak exercise, while these alterations were positively affected when patients were treated with CPAP [8, 29, 30].
ΔPETCO2 max-peak is an objective and reproducible index that is unaffected by influences related to threshold determination and it can be easily measured in any CPET. ROC analysis showed that ΔPETCO2 max-peak was a good predictor of OSA and a cut-off value of 1.71 mmHg can be proposed with a good sensitivity and a fair specificity. However, a reduction of PETCO2 of less than 2 mmHg at peak exercise might be of interest for clinical decision making (sensitivity 74%, specificity 67%). Considering that OSA is more frequent in patients with moderate-severe obesity, this cut-off may help physicians to better interpret CPET values according to patients’ history and symptoms, and to further investigate the potential presence of apnea/hypopnea during sleep, when appropriate.
Limitations and perspectives
In this study, patients’ physical activity level was not recorded. Thus, it could not be excluded that differences in VO2 peak between Ob-OSA and other groups may be due, at least in part, to training levels. Indeed, a proposal for future studies could be the implementation of an objective physical activity monitoring system via accelerometers or at least via a physical activity screening questionnaire.
PETCO2 has been used to indirectly estimate arterial CO2 pressure but these values may not match because of ventilation-perfusion mismatch. However, the exclusion criteria used in this study and the substantial normality of VE/VCO2 slope values should have minimized the risk of such pathological conditions. Future research projects may provide arterial or transcutaneous blood gases measurements to further address these issues.
Conclusion
CPET is a safe and non-invasive evaluation for all patients with chronic diseases, including obesity. Patients with moderate to severe obesity and OSA presented reduced aerobic capacity, exercise tolerance, and ventilatory response with an associated higher PETCO2 at peak exercise. We suggest ΔPETCO2 max-peak as an objective and easily reproducible predictor of OSA. Therefore, outpatient screening with CPET may also provide useful information for the early identification of patients with suspected OSA. Finally, CPET may also be useful for the follow-up of patients with OSA and the evaluation of the effect of CPAP therapy.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- AHI:
-
Apnea–hypopnea index
- AT:
-
Anaerobic threshold
- BMI:
-
Body mass index
- CPAP:
-
Continuous positive airway pressure
- CPET:
-
Cardio-pulmonary exercise testing
- DBP:
-
Diastolic blood pressure
- FEV1:
-
Forced expiratory volume in 1st s
- FVC:
-
Forced vital capacity
- HR:
-
Heart rate
- O2 pulse:
-
Ratio between oxygen uptake and heart rate
- OSA:
-
Obstructive sleep apnea syndrome
- PEF:
-
Peak expiratory flow
- PETCO2 :
-
End-tidal carbon dioxide pressure
- RCP:
-
Respiratory compensation point
- RER:
-
Respiratory exchange ratio
- SBP:
-
Systolic blood pressure
- SpO2 :
-
Blood oxygen saturation with pulse oximetry
- VE:
-
Minute ventilation
- VE/VCO2 :
-
Minute ventilation/carbon dioxide production slope
- VO2 :
-
Oxygen uptake
References
Peppard PE, Hagen EW (2018) The last 25 years of obstructive sleep apnea epidemiology-and the next 25? Am J Respir Crit Care Med 197:310–312. https://doi.org/10.1164/rccm.201708-1614PP
Lévy P, Kohler M, McNicholas WT et al (2015) Obstructive sleep apnoea syndrome. Nat Rev Dis Primers 1(1):1–21. https://doi.org/10.1038/nrdp.2015.15
Mendelson M, Marillier M, Bailly S et al (2018) Maximal exercise capacity in patients with obstructive sleep apnoea syndrome: a systematic review and meta-analysis. Eur Respir J 51(6). https://doi.org/10.1183/13993003.02697-2017
Vanhecke TE, Franklin BA, Zalesin KC et al (2008) Cardiorespiratory fitness and obstructive sleep apnea syndrome in morbidly obese patients. Chest 134(3):539–545. https://doi.org/10.1378/chest.08-0567
Innocenti Bruni G, Gigliotti F, Scano G (2012) Obstructive sleep apnea (OSA) does not affect ventilatory and perceptual responses to exercise in morbidly obese subjects. Respir Physiol Neurobiol 183(3):193–200. https://doi.org/10.1016/j.resp.2012.06.029
Berger M, Kline CE, Cepeda FX et al (2019) Does obstructive sleep apnea affect exercise capacity and the hemodynamic response to exercise? An individual patient data and aggregate meta-analysis. Sleep Med Rev 45:42–53. https://doi.org/10.1016/j.smrv.2019.03.002
Maeder MT, Ammann P, Rickli H et al (2008) N-terminal pro-B-type natriuretic peptide and functional capacity in patients with obstructive sleep apnea. Sleep and Breathing 12(1):7–16. https://doi.org/10.1007/s11325-007-0143-9
Lin CC, Lin CK, Wu KM, Chou CS (2004) Effect of treatment by nasal CPAP on cardiopulmonary exercise test in obstructive sleep apnea syndrome. Lung 182(4):199–212. https://doi.org/10.1007/s00408-004-2502-7
Fletcher HV, Cho PSP, Loong SL et al (2020) Effect of continuous positive airway pressure on maximal exercise capacity in patients with obstructive sleep apnea: a systematic review and meta-analysis. J Clin Sleep Med 16(11):1847–1855. https://doi.org/10.5664/jcsm.8686
Marin JM, Carrizo SJ, Vicente E, Agusti AG (2005) Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. The Lancet 365(9464):1046–1053. https://doi.org/10.1016/s0140-6736(05)71141-7
Berry RB, Budhiraja R, Gottlieb DJ et al (2012) Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med 8(5):597–619. https://doi.org/10.5664/jcsm.2172
Guazzi M, Arena R, Halle M, Piepoli MF, Myers J, Lavie CJ (2016) 2016 focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 133(24):e694–e711. https://doi.org/10.1161/CIR.0000000000000406
Yu L, Li H, Liu X et al (2020) Left ventricular remodeling and dysfunction in obstructive sleep apnea: Systematic review and meta-analysis. Herz 45(8):726–738. https://doi.org/10.1007/s00059-019-04850-w
Li J, Lin X, Li H et al (2020) Right ventricular diastolic dysfunction in patients with obstructive sleep apnea syndrome. Echocardiography 37(2):317–322. https://doi.org/10.1111/echo.14600
Vanuxem D, Badier M, Guillot C, Delpierre S, Jahjah F, Vanuxem P (1997) Impairment of muscle energy metabolism in patients with sleep apnoea syndrome. Respir Med 91(9):551–557. https://doi.org/10.1016/S0954-6111(97)90089-5
Sauleda J, García-Palmer FJ, Tarraga S, Maimó A, Palou A, Agustí AGN (2003) Skeletal muscle changes in patients with obstructive sleep apnoea syndrome. Respir Med 97(7):804–810. https://doi.org/10.1016/S0954-6111(03)00034-9
Temesi J, Arnal PJ, Davranche K et al (2013) Does central fatigue explain reduced cycling after complete sleep deprivation? Med Sci Sports Exerc 45(12):2243–2253. https://doi.org/10.1249/MSS.0b013e31829ce379
Kline CE, Crowley EP, Ewing GB et al (2011) The effect of exercise training on obstructive sleep apnea and sleep quality: a randomized controlled trial. Sleep 34(12):1631–1640. https://doi.org/10.5665/sleep.1422
Verwimp J, Ameye L, Bruyneel M (2013) Correlation between sleep parameters, physical activity and quality of life in somnolent moderate to severe obstructive sleep apnea adult patients. Sleep Breath 17(3):1039–1046. https://doi.org/10.1007/s11325-012-0796-x
Quadri F, Boni E, Pini L et al (2017) Exercise tolerance in obstructive sleep apnea-hypopnea (OSAH), before and after CPAP treatment: effects of autonomic dysfunction improvement. Respir Physiol Neurobiol 236:51–56. https://doi.org/10.1016/j.resp.2016.11.004
Maeder MT, Ammann P, Münzer T et al (2009) Continuous positive airway pressure improves exercise capacity and heart rate recovery in obstructive sleep apnea. Int J Cardiol 132(1):75–83. https://doi.org/10.1016/j.ijcard.2007.10.040
Jing J, Huang T, Cui W, Shen H (2008) Effect on quality of life of continuous positive airway pressure in patients with obstructive sleep apnea syndrome: a meta-analysis. Lung 186(3):131–144. https://doi.org/10.1007/s00408-008-9079-5
Kaleth AS, Chittenden TW, Hawkins BJ et al (2007) Unique cardiopulmonary exercise test responses in overweight middle-aged adults with obstructive sleep apnea. Sleep Med 8(2):160–168. https://doi.org/10.1016/j.sleep.2006.08.005
Nelesen RA, Dimsdale JE, Mills PJ, Clausen JL, Ziegler MG, Ancoli-Israel S (1996) Altered cardiac contractility in sleep apnea. Sleep 19(2):139–144. https://doi.org/10.1093/sleep/19.2.139
Tryfon S, Stanopoulos I, Dascalopoulou E, Argyropoulou P, Bouros D, Mavrofridis E (2004) Sleep apnea syndrome and diastolic blood pressure elevation during exercise. Respiration 71(5):499–504. https://doi.org/10.1159/000080635
Dempsey JA (2005) Crossing the apnoeic threshold: causes and consequences. In: Experimental Physiology. Vol 90. Blackwell Publishing Ltd; 13–24. https://doi.org/10.1113/expphysiol.2004.028985
Kawata N, Tatsumi K, Terada J et al (2007) Daytime hypercapnia in obstructive sleep apnea syndrome. Chest 132(6):1832–1838. https://doi.org/10.1378/chest.07-0673
Magnan A, Philip-Joet F, Rey M, Reynaud M, Porri F, Arnaud A (1993) End-tidal CO2 analysis in sleep apnea syndrome: conditions for use. Chest 103(1):129–131. https://doi.org/10.1378/chest.103.1.129
Stavrou V, Boutou AK, Vavougios GD et al (2019) The use of cardiopulmonary exercise testing in identifying the presence of obstructive sleep apnea syndrome in patients with compatible symptomatology. Respir Physiol Neurobiol 262:26–31. https://doi.org/10.1016/j.resp.2019.01.010
Lin CC, Hsieh WY, Chou CS, Liaw SF (2006) Cardiopulmonary exercise testing in obstructive sleep apnea syndrome. Respir Physiol Neurobiol 150(1):27–34. https://doi.org/10.1016/j.resp.2005.01.008
Acknowledgements
The authors thank the staff of the Sport and Exercise Medicine Division of the Department of Medicine for the valuable support during patients’ evaluations. This research project is part of the Italian initiative of Exercise is Medicine.
Funding
Open access funding provided by Universita degli Studi di Padova within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
All authors participated in the preparation of the manuscript and approved this submission.
Conceptualization: Marco Vecchiato, Andrea Gasperetti. Data curation: Marco Vecchiato, Daniel Neunhaeuserer, Giulia Quinto, Silvia Bettini. Formal analysis: Marco Vecchiato, Giulia Quinto, Francesca Battista. Investigation: Marco Vecchiato, Daniel Neunhaeuserer, Silvia Bettini, Andrea Gasperetti, Andrea Ermolao. Methodology: Marco Vecchiato, Daniel Neunhaeuserer, Francesca Battista, Luca Busetto. Project administration: Andrea Vianello, Roberto Vettor, Luca Busetto, Andrea Ermolao. Supervision: Roberto Vettor, Andrea Ermolao. Writing-original draft: Marco Vecchiato. Writing-review and editing: Marco Vecchiato, Daniel Neunhaeuserer, Giulia Quinto, Silvia Bettini, Andrea Vianello, Luca Busetto, Andrea Ermolao.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in accordance with the Declaration of Helsinki and approved by the local ethics committee (99n/AO/21).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vecchiato, M., Neunhaeuserer, D., Quinto, G. et al. Cardiopulmonary exercise testing in patients with moderate-severe obesity: a clinical evaluation tool for OSA?. Sleep Breath 26, 1115–1123 (2022). https://doi.org/10.1007/s11325-021-02475-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-021-02475-0