Abstract
Purpose
The current established technique for sentinel lymph node (SLN) biopsy is preoperative injection of 99mtechnetium-labeled nanosized colloids (99mTc) followed by single photon emission computed tomography and standard computed tomography (SPECT/CT) with subsequent intraoperative gamma probe-guided excision of the SLN. It is however time and resource consuming, causes radiation exposure and morbidity for the patient as the injection is done in the awake patient. Recently near-infrared imaging with indocyanine green (ICG) gained importance in SLN biopsy as a faster and more convenient technique. The objective of our study was to investigate the feasibility of SLN biopsy using ICG-imaging in early oral squamous cell carcinoma (OSCC).
Methods
Single-centre pilot study of five patients with early-stage OSCC. For all patients, both techniques (99mTc and ICG) were performed. We injected 99mTc preoperatively in the awake patient, followed by SPECT/CT imaging. Intraoperatively ICG was injected around the primary tumor. Then the neck incision was performed according to the SPECT/CT images and SLN were detected by using a gamma probe and near-infrared fluorescence imaging of the ICG-marked lymph nodes intraoperatively. The excised lymph nodes were sent to histopathological examination according to the SLN dissection protocol.
Results
In all five patients sentinel lymph nodes were identified. A total of 7 SLN were identified after injection of 99mTc, imaging with SPECT/CT and intraoperative use of a gamma probe. All these SLN were fluorescent and visible with the ICG technique. In two patients, we could identify additional lymph nodes using the ICG technique. Pathological analysis demonstrated occult metastasis in two of the cases.
Conclusions
Our study shows that ICG-guided SLN biopsy is a feasible technique, especially in combination with conventional radioisotope method and may help for intraoperative localization of SLN. Validation studies with bigger patient cohorts are needed to prove our results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral squamous cell carcinoma (OSCC) is an aggressive malignancy with propensity to local invasion and lymph node metastasis. 20–30% of patients with early-stage OSCC with clinically node-negative lymph node status (cN0) will have occult lymph node metastasis upon pathological examination of the lymphatic drainage [1, 2]. This information is important since regional lymph node metastasis is the most important prognostic factor of OSCC [3,4,5], with a decrease of about 50% in survival when present [6]. There are treatment options for early stage OSCC with a clinically N0 neck. One possibility is to perform a selective neck dissection, which may lead to overtreatment of 70–80% of the patients and morbidity [7]. Typical complications, occurring in under 15% of the cases, include marginal mandibular, vagus, hypoglossal or phrenic nerve injury, injury of the accessory nerve with shoulder pain or dysfunction, hematoma, infection, chyle leak or lymphedema, wound dehiscence and Horner’s syndrome [8]. Watch and wait and perform therapeutic neck dissection for nodal relapse is another possibility which is no longer recommended since it is detrimental to overall survival [9]. The other option is to perform a sentinel lymph node (SLN) biopsy. The concept of SLN biopsy, with identification of the first draining lymph node(s) of the regional lymph node basin was first reported in in 1977 for penile cancer and was further developed in the 1990s for OSCC [10, 11]. SLN biopsy has meanwhile proven to be a safe, effective, and reliable technique with good accuracy and comparable late recurrences and less morbidity in long-term follow-up studies in OSCC when compared to first line selective neck dissection [7, 12,13,14,15].
The current established technique for sentinel lymph node biopsy is preoperative injection of 99mtechnetium-labeled nanosized colloids (99mTc) in the awake patient followed by single photon emission computed tomography and standard computed tomography (SPECT/CT) for localisation of the sentinel lymph nodes preoperatively. Subsequently intraoperative gamma probe-guided excision of the SLN is performed [16]. This technique using radioisotopes is reliable however preoperative injection around the primary tumor is painful for the patient, and the procedure consumes time and resources. As a consequence, the application is limited to cancers of the oral cavity which is accessible in the awake patient. The injection can either be done the same day of the operation requiring precise planning of imaging and surgery or alternatively in a two-step fashion (SPECT/CT some days before the surgery requiring a re-injection of radioisotope on the day of the surgery), which is logistically easier but presents the disadvantage of potential mismatch between the two injections and increasing radiation exposure for the patient and the health care personnel. Finally intraoperative localization can be challenging due to lack of visualization of the tissue and shine-through-effect, especially for floor of mouth lesions [17, 18].
Recently near-infrared imaging with indocyanine green (ICG) gained importance in SLN biopsy as a faster and more convenient technique in OSCC [19, 20]. ICG could be a good alternative to the current technique of SLN biopsy with injection of radioisotopes, as ICG accumulates in the lymph nodes and can be visualised intraoperatively [21]. ICG is a tricarbocyanine iodide with properties of a near-infrared fluorescent dye. Fluorescence of the ICG molecule is normally observed around the maximum peak of 800—850 nm. It is water soluble, has low toxicity and a low molecular weight with quick clearance by hepatic route. It can be injected and traced intraoperatively and enables an intraoperative high-resolution visualisation of the lymph nodes and lymphatic tissue [17, 21, 22]. After injection of the ICG dye in the peritumoral region it takes only a couple of minutes for the ICG to follow the lymphatic vessels and reach the first draining lymph nodes. It provides visual feedback with high area of fluorescence, indicating the precise localisation of the SLN. Especially in cervical cancer and melanoma patients it has been shown to yield good results in sentinel lymph node biopsy [22].
The objective of this study is to investigate the use of near-infrared imaging and ICG in patients with early OSCC and a clinically N0 neck and to confirm its feasibility compared with the conventional radioisotope guided SLN biopsy method.
Material and Methods
Study Design and Patients
In this single-center, non-randomized pilot study we investigated the feasibility of sentinel lymph node detection with ICG technique in patients with early OSCC. After institutional board review (Req-2022–00395) we enrolled five patients with histopathological proven oral cavity malignancy with a T1 or T2 and cN0 stage. Clinical stage was based on physical examination and imaging studies (CT or MRI, neck ultrasound with ultrasound-guided fine needle aspiration biopsy as needed). For all patients, both conventional 99mTc based and ICG based SLN biopsies were performed. Exclusion criteria were pregnancy, allergy to iodine, known intolerance to ICG, previous neck surgery or radiotherapy or recurrent cancer. The study was conducted in accordance with the Declaration of Helsinki. Informed consent for further use of patient related data and material was obtained from all included patients.
SLN Biopsy Technique with 99mTc Radiolabelled Human Albumin Nanocolloid and ICG-Injection
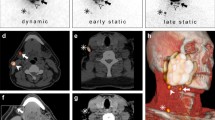
Our head and neck multidisciplinary team (MDT) adheres to the National Comprehensive Cancer Network (NCCN) guidelines [23] for the treatment of our patients. After diagnosis and treatment recommendation by our head and neck MDT a resection of the primary tumor and SLN biopsy was performed in all patients [18]. The SLN biopsy technique with radioisotopes with injection of 99mTc was performed preoperatively in the awake patient by the head and neck surgeon. We injected the 99mTc radiolabelled human albumin nanocolloid (99mTc NanoHSA-ROTOP (ROTOP Pharmaka AG, Dresden, Germany)) at four places into the adjacent mucosa around the primary tumor. A range between 70 and 113 MBq per patient was injected. Immediately after the radiotracer injection lymphoscintigraphy is started to show the drainage pattern and a SPECT/CT is performed by the nuclear medicine specialist. All areas of focal increased uptake outside the primary injection site were considered as SLN (Fig. 1). Then, surgery was carried out about 2–4 h after the SPECT/CT.
In the operating theatre the ICG-dye (Verdye ®, Diagnostic Green GmbH, Germany) was injected around the primary tumor after anesthesia induction with the patient asleep into the same locations as the 99mTc NanoHSA has been injected preoperatively. The ICG-dye is supplied as a water soluble, sterile and powder and was prepared before application with aqua ad iniectabila (2.5 mg/mL, 25 mg of ICG was diluted into 10 ml of aqua ad injectabila). An amount of 1–2 mL of this solution was injected around the tumor. Subsequently excision of the primary tumor and the neck incision was performed according to the preoperative SPECT/CT images which were available in the operating theatre to expose the SLN prior detected on SPECT/CT. The standard subplatysmal flaps were raised. With the thus exposed neck, the area of the sentinel lymph node(s) was detected with the hand-held gamma probe, the sentinel lymph nodes were then made visible with the ICG and near-infrared fluorescence imaging (Fig. 2). As gamma probe we used Neoprobe Gamma Detection System, Model 2300 (Neoprobe Corporation, OH). Time between injection of the ICG and intraoperative visualization of the SLN was between 30 and 60 min. We used the Fluobeam-System (Fluoptics, Grenoble, France) to perform the real-time near-infrared fluorescence imaging and detection of the ICG-marked lymph nodes. To improve the contrast and detection of the lymph nodes, the operating room lights were temporarily dimmed. We only categorized the lymph nodes into fluorescent or non-fluorescent and did not conduct quantification of the fluorescence of the lymph nodes. All detected SLNs—the ones found to have radioactivity and fluorescence as well as the ones found to have fluorescence only—were excised and sent to histopathological examination according to the sentinel lymph node dissection protocol. The SLN were considered as cleared once the surgical bed was free of radiation and ICG-containing nodes, as per screening with the hand-held gamma probe and near-infrared fluorescence imaging.
Results
We included five patients with early OSCC in this pilot study between June 2021 and August 2022. Three of the five patients were male and mean age at surgery was 61.6 years (SD 10.85). Preoperative T stage was cT1 in 3 patients, and cT2 in 2 patients. All the patients had a clinically node-negative lymph node status and no evidence of distant metastasis preoperatively. In all patients we conducted a radical resection of the primary tumor with negative surgical margins after injection of 99mTc and ICG. In all patients primary closure was possible with no need for surgical reconstruction. In three of the five patients, the T category had to be upstaged postoperatively due to depth of invasion or size of greatest dimension. In two patients occult lymph node metastasis was found in one SLN. Both histopathological positive SLN were detected with ICG and the radioisotope method. Figure 3 shows the histopathological image of the metastasis found in the SLN of Patient 5. Further patients baseline characteristics are described in Table 1.
The SLN could not be visualized transcutaneous with near-infrared fluorescence imaging in all five patients. After performing the standard subplatysmal flaps according to the preoperative SPECT/CT the SLN were made visible with ICG and were detected with the hand held gamma probe. Table 2 shows the number of sentinel lymph nodes detected by the two techniques used. At least one SLN was detected in all patients. With the radioisotope technique, we identified 7 sentinel lymph nodes. Intraoperatively all SLN identified with the radioisotope technique were also fluorescent and detected with ICG technique. The ICG injection around the periphery of the primary tumor intraoperatively and near-infrared fluorescence imaging after skin incision and initial tissue preparation of the neck, allowed the identification of two additional fluorescent lymph nodes, resulting in a total of 9 excised lymph nodes. In both of the two additional detected lymph nodes no occult metastasis was found. A mean of 1.8 (SD 0.45) lymph nodes was found in each patient. All the SLN were detected in the ipsilateral neck. Intraoperatively we performed a frozen section in two patients. In patient 2 the frozen section and definitive histopathology did not show any occult metastasis. In patient 4 the lymph node metastasis was diagnosed on frozen section and a unilateral neck dissection of the levels I to III was performed during the same anesthesia. In patient 5 a staged completion unilateral neck dissection of the levels I to IV was performed after detection of a metastasis in the sentinel lymph node on final histopathology. There were no adverse effects from the use of the ICG-dye intraoperatively. There were also no surgical complications noted during our pilot study.
During the follow-up time three of the five included patients were tumor-free, one died due-to another malignancy and one patient showed a local recurrence on the tongue.
Discussion
This was a single-center pilot study with five OSCC patients undergoing SLN biopsy with conventional radioisotope technique and ICG-technique, which showed that SLN biopsy with ICG near-infrared imaging is basically a feasible and reliable method to detect the SLN in early OSCC patients.
In our study with the ICG-technique we found two lymph nodes in addition to the 7 SLN detected by the conventional radioisotope technique, this concerts with to the results by Nakamura et al. [19]. Christensen et al. [24] showed that the majority of the additional detected lymph nodes with ICG were located in level I, close to the primary tumor. The detection of SLN with use of the gamma-probe can be difficult due to the shine-trough-effect especially in floor of mouth lesions. The average number of SLN per patient was 1.8, which was a bit lower than in previous studies who detected a mean of 2—3.4 SLN with ICG [19, 25, 26]. Our results and the current literature suggest that the additional usage of ICG and near-infrared imaging in combination with radiotracers simplifies and increases the intraoperative detection of the SLN through better visualization and resolution via ICG and this therefore allows to identify more lymph nodes than with the radioisotope method alone [19, 24, 27]. Additionally Nakamura et al. showed a tendency of shorter operating time when adding the ICG tracer for intraoperative detection [19]. This applies especially where shine-trough-effects can lead to difficulty of detection of the SLN with the use of a gamma-probe [24, 27].
In two of the five patients we found occult lymph node metastasis in one of the excised SLN, where in the other three patients SNL biopsies were negative on histopathology. This corresponds to the knowledge that that in up to 30% of early OSCC occult metastasis can be found and that SLN biopsies can detect these occult metastasis [2, 28].
ICG is a small molecule and binds intravascularly to plasma proteins, mostly albumin and alpha- and beta-lipoproteins and is transported quickly to the SLN, this allows for the quick visualization of the SLN within a few minutes after injection, and the ICG-injection to be done after anesthesia induction with the patient asleep thus avoiding the pain morbidity related to the injection in the awake patient [19]. The molecule size, however, is an important factor for the distribution with smaller molecules being transported and distributed faster potentially also into higher echelon nodes. This may lead to a overestimation of the number of (sentinel) lymph nodes detected with ICG [19], corresponding to our finding of two additional detected lymph nodes with near-infrared fluorescence imaging, compared with the radioisotope method. Especially with longer time between injection and detection of the lymph nodes [21]. Another factor for optimal visibility is the concentration and volume of the injected ICG. A Meta-analysis from 2014 suggested improved lymphatic drainage imaging with lower concentration and larger volume to the injected ICG-solution [29]. Furthermore Markuszewski et al. showed that the quality of visualization correlates with the ratio of albumin and ICG. If there is an excess of ICG, free dye is present what results in imprecise images of the SLN. And that higher and longer luminescence could be achieved with use of ICG bound with a human serum albumin [30]. Another possibility is to use hybrid tracer (ICG-99mTc-nanocolloid) to avoid the transport of the ICG-tracer to higher echelon lymph nodes or leakage into the surgical field [31]. It is known that an ideal tracer should combine rapid transport and durable retention in the SLN, and that the distribution of the injected particles depends on the particle size and stability of the administered colloid particles as it was studied for radionuclides in the context of SLN biopsy. Therefore, no valid SLN biopsy can be performed by using small particles (smaller than 4 – 5 nm), as they penetrate the capillary membranes and therefore are not migrating trough the lymphatic channels and are dispersed into the vascular system. Neither with usage of large particles (500 – 2000 nm), as they stay trapped at the injection site and do not enter the lymphatic system [32, 33]. We used 99mTc NanoHSA, i.e. radionuclide bound to human serum albumin with a particle size that drain fast into the lymph nodes and accumulate there within 10 min and are large enough to stay in the first draining nodes and not to overflow in higher echelon lymph nodes [2]. In more than 95% the particles of the nanocolloidal albumin are smaller than 80 nm, around 4% have a size of 80 – 100 nm and only 1% is larger than 100 nm [32, 33]. Further studies for better understanding in the transportation and distribution of the ICG-molecules and for determination of the best concentration and amount of injected ICG-solution are needed.
ICG as a new tracer and near-infrared imaging for detection of SLN has gained importance in recent years. ICG is free of radiation and has almost no side effects and risks. We were also able to confirm this in our study, as no side effects occurred after peritumoral injection of the ICG. There is no need for preoperative injection as this method is a one-step procedure with injection of the primary lesion during anesthesia which makes this technique of SLN biopsy more convenient and far less morbid for the patient. Other advantages of SLN biopsy with ICG are the lower costs of the tracer compared with radiotracers, no need for a nuclear physician and no exposure of the patient and medical personnel to radiation [25]. In addition, the shine-through-effect which typically occurs in radioisotope SLN mapping for floor of mouth cancers does not exist with the ICG-SLN biopsy [19, 25].
With the possibility of the intraoperative injection of the ICG under general anesthesia, the use of the ICG and therefore SLN biopsy could potentially be extended to other tumor sites of the head and neck such as laryngeal, hypopharyngeal or thyroid malignancies which are currently not accessible for preoperative injection with radioisotopes and subsequent SLN biopsy.
One of the limitations of our study was and of the usage of ICG for SLN biopsy in general is, that we were not able to visualize the SLN by ICG and near-infrared imaging through the skin without a neck incision. It is known that current disadvantages of the ICG imaging are its limited depth of penetration of 0.5 – 1.5 cm through the skin and subcutaneous tissue [34, 35]. This means that the lymph nodes cannot always be visualized transcutaneously without previous neck incision. Especially in OSCC, where the lymphatic channels and the first draining lymph nodes are usually located in the deep fatty tissue of the neck and are often blocked by the mandible or the sternocleidomastoid muscle. This has also been shown in a study from 2012, where only 12% of the SLN were detectable with only near-infrared imaging and the majority of these detected SLN with ICG only were located in Level I, where the shine through effect affects detectability of the sentinel lymph nodes with the radioisotope method [24], However, Nakamura et al. showed good transcutaneous visualization of the sentinel lymph nodes by applying pressure on the skin [19]. One possible factor for better visualization transcutaneously could be the BMI of the individual patient as Christensen et al. showed an association with lower BMI successful visualization transcutaneously [24].
As a result of these disadvantages of the ICG-method, the above-mentioned advantages of the ICG (no pain for the patient during injection, no need for a nuclear specialist, no radiation exposure, lesser costs) are not yet as significant, as for the moment the ICG can only be used in combination with the radioisotope method for SLN biopsy in OSCC.
Another limitation of our study is the small number of patients included as this was a pilot study to investigate the feasibility of the ICG-technique for SLN in head and neck tumors.
In future, ICG-guided sentinel lymph node biopsy could be used in a complementary fashion for faster intraoperative detection and better visualization of lymph nodes in addition to the current SLN biopsy with preoperative injection of radioisotopes and SPECT/CT. With technical progress and better transcutaneous visualization the ICG-guided sentinel lymph node biopsy could possible replace the conventional SLN biopsy in the future, but this needs to be validated with prospective studies which are currently underway at our unit.
Conclusion
We demonstrated that SLN biopsy with ICG near-infrared imaging is a feasible and valid method for detection of sentinel lymph nodes, especially in combination with current state of the art method with preoperative injection of radioisotope and SPECT/CT. The ICG technique provides a better and faster visualization and thus easier intraoperative detection of the SLN. The ICG-SLN technique could be extended to other regions of the head and neck which are currently not accessible through injection in the awake patient.
Data Availability
The datasets generated for this study can be obtained upon reasonable request by email to the corresponding author.
References
Morand GB, Ikenberg K, Vital DG, Cardona I, Moch H, Stoeckli SJ, Huber GF (2019) Preoperative assessment of CD44-mediated depth of invasion as predictor of occult metastases in early oral squamous cell carcinoma. Head Neck 41(4):950–958
Stoeckli SJ (2007) Sentinel node biopsy for oral and oropharyngeal squamous cell carcinoma of the head and neck. Laryngoscope 117(9):1539–1551
Sievert M, Mantsopoulos K, Iro H, Koch M (2021) Near-infrared sentinel diagnostics in head and neck squamous cell carcinoma: a systematic review. Laryngorhinootologie 101(5):383–389. German
Miura K, Hirakawa H, Uemura H, Yoshimoto S, Shiotani A, Sugasawa M, Homma A, Yokoyama J, Tsukahara K, Yoshizaki T et al (2017) Sentinel node biopsy for oral cancer: A prospective multicenter phase II trial. Auris Nasus Larynx 44(3):319–326
Pantel K, Brakenhoff RH (2004) Dissecting the metastatic cascade. Nat Rev Cancer 4(6):448–456
Shah JP, Gil Z (2009) Current concepts in management of oral cancer–surgery. Oral Oncol 45(4–5):394–401
Murer K, Huber GF, Haile SR, Stoeckli SJ (2011) Comparison of morbidity between sentinel node biopsy and elective neck dissection for treatment of the n0 neck in patients with oral squamous cell carcinoma. Head Neck 33(9):1260–1264
Dunlap Q, Gardner JR, Ederle A, King D, Merriweather M, Vural E, Moreno MA (2022) Comparative morbidity profile of elective vs therapeutic neck dissection. Otolaryngol Head Neck Surg 166(2):327–333
D’Cruz AK, Vaish R, Kapre N, Dandekar M, Gupta S, Hawaldar R, Agarwal JP, Pantvaidya G, Chaukar D, Deshmukh A et al (2015) Elective versus therapeutic neck dissection in node-negative oral cancer. N Engl J Med 373(6):521–529
Alex JC, Krag DN (1996) The gamma-probe-guided resection of radiolabeled primary lymph nodes. Surg Oncol Clin N Am 5(1):33–41
de Bree R, Nieweg OE (2015) The history of sentinel node biopsy in head and neck cancer: From visualization of lymphatic vessels to sentinel nodes. Oral Oncol 51(9):819–823
Muhanna N, Chan HHL, Douglas CM, Daly MJ, Jaidka A, Eu D, Bernstein J, Townson JL, Irish JC (2020) Sentinel lymph node mapping using ICG fluorescence and cone beam CT - a feasibility study in a rabbit model of oral cancer. BMC Med Imaging 20(1):106
Broglie MA, Haile SR, Stoeckli SJ (2011) Long-term experience in sentinel node biopsy for early oral and oropharyngeal squamous cell carcinoma. Ann Surg Oncol 18(10):2732–2738
Moya-Plana A, Auperin A, Guerlain J, Gorphe P, Casiraghi O, Mamelle G, Melkane A, Lumbroso J, Janot F, Temam S (2018) Sentinel node biopsy in early oral squamous cell carcinomas: Long-term follow-up and nodal failure analysis. Oral Oncol 82:187–194
Schilling C, Stoeckli SJ, Haerle SK, Broglie MA, Huber GF, Sorensen JA, Bakholdt V, Krogdahl A, von Buchwald C, Bilde A et al (2015) Sentinel European Node Trial (SENT): 3-year results of sentinel node biopsy in oral cancer. Eur J Cancer 51(18):2777–2784
Haerle SK, Hany TF, Strobel K, Sidler D, Stoeckli SJ (2009) Is there an additional value of SPECT/CT over planar lymphoscintigraphy for sentinel node mapping in oral/oropharyngeal squamous cell carcinoma? Ann Surg Oncol 16(11):3118–3124
Zeng HC, Hu JL, Bai JW, Zhang GJ (2019) Detection of Sentinel Lymph Nodes with Near-Infrared Imaging in Malignancies. Mol Imaging Biol 21(2):219–227
Stoeckli SJ, Alkureishi LW, Ross GL (2009) Sentinel node biopsy for early oral and oropharyngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol 266(6):787–793
Nakamura T, Kogashiwa Y, Nagafuji H, Yamauchi K, Kohno N (2015) Validity of sentinel lymph node biopsy by ICG fluorescence for early head and neck cancer. Anticancer Res 35(3):1669–1674
Al-Dam A, Precht C, Barbe A, Kohlmeier C, Hanken H, Wikner J, Schön G, Heiland M, Assaf AT (2018) Sensitivity and specificity of sentinel lymph node biopsy in patients with oral squamous cell carcinomas using indocyanine green fluorescence imaging. J Craniomaxillofac Surg 46(8):1379–1384
Yokoyama J, Hasegawa Y, Sugasawa M, Shiotani A, Murakami Y, Ohba S, Kohno N (2020) Long term-follow-up multicenter feasibility study of ICG fluorescence-navigated sentinel node biopsy in oral cancer. Mol Clin Oncol 13(4):41
Reinhart MB, Huntington CR, Blair LJ, Heniford BT, Augenstein VA (2016) Indocyanine Green: Historical Context, Current Applications, and Future Considerations. Surg Innov 23(2):166–175
Pfister DG, Spencer S, Adelstein D, Adkins D, Anzai Y, Brizel DM, Bruce JY, Busse PM, Caudell JJ, Cmelak AJ et al (2020) Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 18(7):873–898
Christensen A, Juhl K, Charabi B, Mortensen J, Kiss K, Kjaer A, von Buchwald C (2016) Feasibility of real-time near-infrared fluorescence tracer imaging in sentinel node biopsy for oral cavity cancer patients. Ann Surg Oncol 23(2):565–572
Peng H, Wang SJ, Niu X, Yang X, Chi C, Zhang G (2015) Sentinel node biopsy using indocyanine green in oral/oropharyngeal cancer. World J Surg Oncol 13:278
Hingsammer L, Schönegg D, Gander T, Lanzer M (2023) Radioactive nanosized colloids and indocyanine green identify the same sentinel lymph nodes in oral squamous cell carcinoma. J Cancer Res Clin Oncol 149(19):17223–17229
van den Berg NS, Brouwer OR, Klop WM, Karakullukcu B, Zuur CL, Tan IB, Balm AJ, van den Brekel MW, Valdes Olmos RA, van Leeuwen FW (2012) Concomitant radio- and fluorescence-guided sentinel lymph node biopsy in squamous cell carcinoma of the oral cavity using ICG-(99m)Tc-nanocolloid. Eur J Nucl Med Mol Imaging 39(7):1128–1136
Melkane AE, Mamelle G, Wycisk G, Temam S, Janot F, Casiraghi O, Lumbroso J (2012) Sentinel node biopsy in early oral squamous cell carcinomas: a 10-year experience. Laryngoscope 122(8):1782–1788
Xiong L, Gazyakan E, Yang W, Engel H, Hunerbein M, Kneser U, Hirche C (2014) Indocyanine green fluorescence-guided sentinel node biopsy: a meta-analysis on detection rate and diagnostic performance. Eur J Surg Oncol 40(7):843–849
Markuszewski M, Buszewska-Forajta M, Artymowicz M, Polom W, Roslan M, Markuszewski M (2022) Binding indocyanine green to human serum albumin potentially enhances the detection of sentinel lymph nodes. An initial step for facilitating the detection of first-station nodes in penile and other urological cancers. Arch Med Sci 18(3):719–725
KleinJan GH, van Werkhoven E, van den Berg NS, Karakullukcu MB, Zijlmans H, van der Hage JA, van de Wiel BA, Buckle T, Klop WMC, Horenblas S et al (2018) The best of both worlds: a hybrid approach for optimal pre- and intraoperative identification of sentinel lymph nodes. Eur J Nucl Med Mol Imaging 45(11):1915–1925
Wilhelm AJ, Mijnhout GS, Franssen EJ (1999) Radiopharmaceuticals in sentinel lymph-node detection - an overview. Eur J Nucl Med 26(4 Suppl):S36-42
Ahmed M, Purushotham AD, Douek M (2014) Novel techniques for sentinel lymph node biopsy in breast cancer: a systematic review. Lancet Oncol 15(8):e351-362
Kim JH, Ku M, Yang J, Byeon HK (2021) Recent developments of ICG-guided sentinel lymph node mapping in oral cancer. Diagnostics (Basel) 11(5):891
Benedict PA, Zhou L, Peng R, Kohan D (2018) The malleus to oval window revision stapedotomy: Efficacy and longitudinal study outcome. Laryngoscope 128(2):461–467
Acknowledgements
The authors would like to thank the Department of Pathology and Nuclear Medicine at our center for providing the histopathological and radiological images.
Funding
Open access funding provided by University of Luzern This study did not receive any funding.
Author information
Authors and Affiliations
Contributions
Study concept by NS and GR. Data collection and curation by NS. Manuscript drafting by NS. Statistics by NS and GBM. Manuscript editing and review by all co-authors. All authors have participated substantially to the study and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical Standards
This study received institutional review board approvals at all involved institutions.
Consent for Publication
All authors approved the final version of the manuscript and consented to its publication in its actual form.
Conflicts of Interest
All the authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stenz, N.A., Morand, G.B., Schoch, M. et al. Use of Indocyanine Green Near-Infrared Imaging for Sentinel Lymph Node Biopsy in Early Oral Squamous Cell Carcinoma: A Pilot Study. Mol Imaging Biol 26, 264–271 (2024). https://doi.org/10.1007/s11307-024-01903-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-024-01903-3