Abstract
Purpose
Our objectives were to develop a targeted microbubble with an anti-P-selectin aptamer and assess its ability to detect bowel inflammation in two murine models of acute colitis.
Procedures.
Lipid-shelled microbubbles were prepared using mechanical agitation. A rapid copper-free click chemistry approach (azide-DBCO) was used to conjugate the fluorescent anti-P-selectin aptamer (Fluor-P-Ap) to the microbubble surface. Bowel inflammation was chemically induced using 2,4,6-trinitrobenzenesulfonic acid (TNBS) in both Balb/C and interleukin-10-deficient (IL-10 KO) mice. Mouse bowels were imaged using non-linear contrast mode following an i.v. bolus of 1 × 108 microbubbles. Each mouse received a bolus of aptamer-functionalized and non-targeted microbubbles. Mouse phenotypes and the presence of P-selectin were validated using histology and immunostaining, respectively.
Results
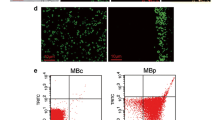
Microbubble labelling of Fluor-P-Ap was complete after 20 min at 37 ̊C. We estimate approximately 300,000 Fluor-P-Ap per microbubble and confirmed fluorescence using confocal microscopy. There was a significant increase in ultrasound molecular imaging signal from both Balb/C (p = 0.003) and IL-10 KO (p = 0.02) mice with inflamed bowels using aptamer-functionalized microbubbles in comparison to non-targeted microbubbles. There was no signal in healthy mice (p = 0.4051) using either microbubble.
Conclusions
We constructed an aptamer-functionalized microbubble specific for P-selectin using a clinically relevant azide-DBCO click reaction, which could detect bowel inflammation in vivo. Aptamers have potential as a next generation targeting agent for developing cost-efficient and clinically translatable targeted microbubbles.






Similar content being viewed by others
References
Ng SC et al (2017) Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. The Lancet 390(10114):2769–2778
Kaplan GG (2015) The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol 12(12):720–727
Rameshshanker R, Arebi N (2012) Endoscopy in inflammatory bowel disease when and why. World J Gastrointest Endosc 4(6):201–211
Maaser C et al (2019) ECCO-ESGAR guideline for diagnostic assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis 13(2):144–164
Panes J et al (2013) Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis 7(7):556–585
Kim M, Jang HJ (2016) The role of small bowel endoscopy in small bowel Crohn’s disease: when and how? Intest Res 14(3):211–217
Dambha F, Tanner J, Carroll N (2014) Diagnostic imaging in Crohn’s disease: what is the new gold standard? Best Pract Res Clin Gastroenterol 28(3):421–436
Yoon HM et al (2017) Diagnostic performance of magnetic resonance enterography for detection of active inflammation in children and adolescents with inflammatory bowel disease: a systematic review and diagnostic meta-analysis. JAMA Pediatr 171(12):1208–1216
Goodsall TM et al (2021) Systematic review: patient perceptions of monitoring tools in inflammatory bowel disease. J Can Assoc Gastroenterol 4(2):e31–e41
Allocca M et al (2019) Noninvasive multimodal methods to differentiate inflamed vs fibrotic strictures in patients with Crohn’s disease. Clin Gastroenterol Hepatol 17(12):2397–2415
Bryant RV et al (2018) Gastrointestinal ultrasound in inflammatory bowel disease: an underused resource with potential paradigm-changing application. Gut 67(5):973–985
Allocca M et al (2021) Point-of-care ultrasound in inflammatory bowel disease. J Crohns Colitis 15(1):143–151
Alkim C et al (2015) Angiogenesis in inflammatory bowel disease. Int J Inflam 2015:970890
Medellin A, Merrill C, Wilson SR (2018) Role of contrast-enhanced ultrasound in evaluation of the bowel. Abdom Radiol (NY) 43(4):918–933
Cosgrove D, Lassau N (2010) Imaging of perfusion using ultrasound. Eur J Nucl Med Mol Imaging 37(Suppl 1):S65-85
Ripolles T et al (2009) Crohn disease: correlation of findings at contrast-enhanced US with severity at endoscopy. Radiology 253(1):241–248
Ripolles T et al (2011) Contrast-enhanced ultrasound (CEUS) in Crohn’s disease: technique, image interpretation and clinical applications. Insights Imaging 2(6):639–652
Ripolles T et al (2013) Effectiveness of contrast-enhanced ultrasound for characterisation of intestinal inflammation in Crohn’s disease: a comparison with surgical histopathology analysis. J Crohns Colitis 7(2):120–128
Kucharzik T, Kannengiesser K, Petersen F (2017) The use of ultrasound in inflammatory bowel disease. Ann Gastroenterol 30(2):135–144
Kucharzik T, Maaser C (2018) Intestinal ultrasound and management of small bowel Crohn’s disease. Therap Adv Gastroenterol 11:1756284818771367
Klibanov AL (2007) Ultrasound molecular imaging with targeted microbubble contrast agents. J Nucl Cardiol 14(6):876–884
Deshpande N, Needles A, Willmann JK (2010) Molecular ultrasound imaging: current status and future directions. Clin Radiol 65(7):567–581
James ML, Gambhir SS (2012) A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev 92(2):897–965
Willmann JK et al (2008) Targeted microbubbles for imaging tumor angiogenesis: assessment of whole-body biodistribution with dynamic micro-PET in mice. Radiology 249(1):212–219
Willmann JK et al (2008) Dual-targeted contrast agent for US assessment of tumor angiogenesis in vivo. Radiology 248(3):936–944
Willmann JK et al (2008) US imaging of tumor angiogenesis with microbubbles targeted to vascular endothelial growth factor receptor type 2 in mice. Radiology 246(2):508–518
Wang S et al (2016) Ultra-low-dose ultrasound molecular imaging for the detection of angiogenesis in a mouse murine tumor model: how little can we see? Invest Radiol 51(12):758–766
Slagle CJ et al (2018) Click conjugation of cloaked peptide ligands to microbubbles. Bioconjug Chem 29(5):1534–1543
Zhang H et al (2015) Ultrasound molecular imaging of tumor angiogenesis with a neuropilin-1-targeted microbubble. Biomaterials 56:104–113
Bachmann C et al (2006) Targeting mucosal addressin cellular adhesion molecule (MAdCAM)-1 to noninvasively image experimental Crohn’s disease. Gastroenterology 130(1):8–16
Deshpande N et al (2012) Quantification and monitoring of inflammation in murine inflammatory bowel disease with targeted contrast-enhanced US. Radiology 262(1):172–180
El Kaffas A et al (2017) Molecular Contrast-enhanced ultrasound imaging of radiation-induced p-selectin expression in healthy mice colon. Int J Radiat Oncol Biol Phys 97(3):581–585
Ahmed M et al (2019) Molecular imaging of a new multimodal microbubble for adhesion molecule targeting. Cell Mol Bioeng 12(1):15–32
Lindner JR et al (2001) Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation 104(17):2107–2112
Lindner LR et al (2000) Noninvasive ultrasound imaging of inflammation using microbubbles targeted to activated leukocytes. Circulation 102:2745–2750
Tlaxca JL et al (2013) Ultrasound-based molecular imaging and specific gene delivery to mesenteric vasculature by endothelial adhesion molecule targeted microbubbles in a mouse model of Crohn’s disease. J Control Release 165(3):216–225
Machtaler S et al (2015) Assessment of inflammation in an acute on chronic model of inflammatory bowel disease with ultrasound molecular imaging. Theranostics 5(11):1175–1186
Xie F et al (2009) Diagnostic ultrasound combined with glycoprotein IIb/IIIa-targeted microbubbles improves microvascular recovery after acute coronary thrombotic occlusions. Circulation 119(10):1378–1385
Dayton PA et al (2004) Ultrasonic analysis of peptide- and antibody-targeted microbubble contrast agents for molecular imaging of alphavbeta3-expressing cells. Mol Imaging 3(2):125–134
Leong-Poi H et al (2003) Noninvasive assessment of angiogenesis by ultrasound and microbubbles targeted to alpha(v)-integrins. Circulation 107(3):455–460
Willmann JK et al (2010) Targeted contrast-enhanced ultrasound imaging of tumor angiogenesis with contrast microbubbles conjugated to integrin-binding knottin peptides. J Nucl Med 51(3):433–440
Bam R et al (2020) Efficacy of affibody-based ultrasound molecular imaging of vascular B7–H3 for breast cancer detection. Clin Cancer Res 26(9):2140–2150
Abou-Elkacem L et al (2016) Ultrasound molecular imaging of the breast cancer neovasculature using engineered fibronectin scaffold ligands: a novel class of targeted contrast ultrasound agent. Theranostics 6(11):1740–1752
Abou-Elkacem L et al (2018) Thy1-targeted microbubbles for ultrasound molecular imaging of pancreatic ductal adenocarcinoma. Clin Cancer Res 24(7):1574–1585
Punjabi M et al (2019) Ultrasound molecular imaging of atherosclerosis with nanobodies: translatable microbubble targeting murine and human VCAM (vascular cell adhesion molecule) 1. Arterioscler Thromb Vasc Biol 39(12):2520–2530
Yeh JS et al (2015) A targeting microbubble for ultrasound molecular imaging. PLoS ONE 10(7):e0129681
Alonso A et al (2007) Molecular imaging of human thrombus with novel abciximab immunobubbles and ultrasound. Stroke 38(5):1508–1514
Hernot S et al (2012) Nanobody-coupled microbubbles as novel molecular tracer. J Control Release 158(2):346–353
Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346(6287):818–822
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249(4968):505–510
Yuce M, Ullah N, Budak H (2015) Trends in aptamer selection methods and applications. Analyst 140(16):5379–5399
Bouchard PR, Hutabarat RM, Thompson KM (2010) Discovery and development of therapeutic aptamers. Annu Rev Pharmacol Toxicol 50:237–257
Zhu Q, Liu G, Kai M (2015) DNA aptamers in the diagnosis and treatment of human diseases. Molecules 20(12):20979–20997
Lakhin AV, Tarantul VZ, Gening LV (2013) Aptamers: problems, solutions and prospects. Acta Naturae 5(4):34–43
Nakatsuka MA et al (2013) In vivo ultrasound visualization of non-occlusive blood clots with thrombin-sensitive contrast agents. Biomaterials 34(37):9559–9565
Nakatsuka MA et al (2012) Aptamer-crosslinked microbubbles: smart contrast agents for thrombin-activated ultrasound imaging. Adv Mater 24(45):6010–6016
Gutsaeva DR et al (2011) Inhibition of cell adhesion by anti-P-selectin aptamer: a new potential therapeutic agent for sickle cell disease. Blood 117(2):727–735
Talu E et al (2006) Long-term stability by lipid coating monodisperse microbubbles formed by a flow-focusing device. Langmuir 22(23):9487–9490
Kaya M, Gregory TSt, and Dayton PA (2009) Changes in lipid-encapsulated microbubble population during continuous infusion and methods to maintain consistency Ultrasound Med Biol. 35(10): p. 1748-55
Seo M et al (2010) Microfluidic assembly of monodisperse, nanoparticle-incorporated perfluorocarbon microbubbles for medical imaging and therapy. Langmuir 26(17):13855–13860
Goncin U et al (2022) Rapid copper-free click conjugation to lipid-shelled microbubbles for ultrasound molecular imaging of murine bowel inflammation. Bioconjug Chem 33(5):848–857
Wirtz S et al (2007) Chemically induced mouse models of intestinal inflammation. Nat Protoc 2(3):541–546
Histology Core Facility, UoS, H&E Staining Protocol (2019). https://healthsciences.usask.ca/documents/histology-documents/HandE-April-2019.pdf
Hong H et al (2011) Molecular imaging with nucleic acid aptamers. Curr Med Chem 18(27):4195–4205
Maul TM et al (2010) Optimization of ultrasound contrast agents with computational models to improve selection of ligands and binding strength. Biotechnol Bioeng 107(5):854–864
Wang CH, Huang YF, Yeh CK (2011) Aptamer-conjugated nanobubbles for targeted ultrasound molecular imaging. Langmuir 27(11):6971–6976
Borden MA, Longo ML (2002) Dissolution behavior of lipid monolayer-coated, air-filled microbubbles: effect of lipid hydrophobic chain length. Langmuir 18(24):9225–9233
Borden MA et al (2004) Surface phase behavior and microstructure of lipid/PEG-emulsifier monolayer-coated microbubbles. Colloids Surf B Biointerfaces 35(3–4):209–223
Chen CC, Borden MA (2010) Ligand conjugation to bimodal poly(ethylene glycol) brush layers on microbubbles. Langmuir 26(16):13183–13194
Chen CC, Borden MA (2011) The role of poly(ethylene glycol) brush architecture in complement activation on targeted microbubble surfaces. Biomaterials 32(27):6579–6587
Wang H, et al (2022) Contrast Enhanced ultrasound molecular imaging of spontaneous chronic inflammatory bowel disease in an interleukin-2 receptor alpha(-/-) transgenic mouse model using targeted microbubbles. Nanomaterials (Basel). 12(2)
Wang H et al (2019) Chronic model of inflammatory bowel disease in IL-10(-/-) transgenic mice: evaluation with ultrasound molecular imaging. Theranostics 9(21):6031–6046
Dothel G et al (2013) Animal models of chemically induced intestinal inflammation: predictivity and ethical issues. Pharmacol Ther 139(1):71–86
te Velde AA, Verstege MI, Hommes DW (2006) Critical appraisal of the current practice in murine TNBS-induced colitis. Inflamm Bowel Dis 12(10):995–999
Antoniou E et al (2016) The TNBS-induced colitis animal model: an overview. Ann Med Surg (Lond) 11:9–15
Jones-Hall YL, Grisham MB (2014) Immunopathological characterization of selected mouse models of inflammatory bowel disease: comparison to human disease. Pathophysiology 21(4):267–288
Takai T (2002) Roles of Fc receptors in autoimmunity. Nat Rev Immunol 2(8):580–592
Smith KG, Clatworthy MR (2010) FcgammaRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat Rev Immunol 10(5):328–343
Wilkens R et al (2018) Persistent enhancement on contrast-enhanced ultrasound studies of severe crohn’s disease: stuck bubbles? Ultrasound Med Biol 44(11):2189–2198
Bzyl J et al (2011) Molecular and functional ultrasound imaging in differently aggressive breast cancer xenografts using two novel ultrasound contrast agents (BR55 and BR38). Eur Radiol 21(9):1988–1995
Tardy I et al (2010) Ultrasound molecular imaging of VEGFR2 in a rat prostate tumor model using BR55. Invest Radiol 45(10):573–578
Acknowledgements
We would like to acknowledge that BioRender.com was used to create the Fig. 1.
Funding
This work was supported by a Saskatchewan Health Research Foundation Establishment Grant.
Author information
Authors and Affiliations
Contributions
Una Goncin: resources, conception, investigation, formal analysis, interpretation, writing — original draft, writing — review & editing, approval. Laura Curiel: investigation, interpretation, writing, review. C Ronald Geyer: resources/acquisition, conception, writing — review & editing, approval. Steven Machtaler: resources, conception, interpretation, writing — review & editing, approval.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Goncin, U., Curiel, L., Geyer, C.R. et al. Aptamer-Functionalized Microbubbles Targeted to P-selectin for Ultrasound Molecular Imaging of Murine Bowel Inflammation. Mol Imaging Biol 25, 283–293 (2023). https://doi.org/10.1007/s11307-022-01755-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-022-01755-9