Abstract
Purpose
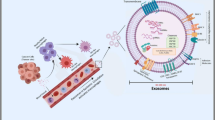
Acidity can be a useful alternative biomarker for the targeting of metabolically active cells in certain diseased tissues, as in acute inflammation or aggressive tumors. We investigated the targeting of activated macrophages by pH low insertion peptides (pHLIPs), an established technology for targeting cell-surface acidity.
Procedures
The uptake of fluorescent pHLIPs by activated macrophages was studied in cell cultures, in a mouse model of lung inflammation, and in a mouse tumor model. Fluorescence microscopy, whole-body and organ imaging, immunohistochemistry, and FACS analysis were employed.
Results
We find that cultured, activated macrophages readily internalize pHLIPs. The uptake is higher in glycolytic macrophages activated by LPS and INF-γ compared to macrophages activated by IL-4/IL-13. Fluorescent pHLIPs target LPS-induced lung inflammation in mice. In addition to marking cancer cells within the tumor microenvironment, fluorescent pHLIPs target CD45+, CD11b+, F4/80+, and CD206+ tumor-associated macrophages with no significant targeting of other immune cells. Also, fluorescent pHLIPs target CD206-positive cells found in the inguinal lymph nodes of animals inoculated with breast cancer cells in mammary fat pads.
Conclusions
pHLIP peptides sense low cell surface pH, which triggers their insertion into the cell membrane. Unlike cancerous cells, activated macrophages do not retain inserted pHLIPs on their surfaces, instead their highly active membrane recycling moves the pHLIPs into endosomes. Targeting activated macrophages in diseased tissues may enable clinical visualization and therapeutic opportunities.






Similar content being viewed by others
References
Parisi L, Gini E, Baci D et al (2018) Macrophage polarization in chronic inflammatory diseases: killers or builders? J Immunol Res 2018:8917804
Davies LC, Jenkins SJ, Allen JE, Taylor PR (2013) Tissue-resident macrophages. Nat Immunol 14:986–995
Murray PJ, Wynn TA (2011) Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11:723–737
Murray PJ, Allen JE, Biswas SK et al (2014) Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41:14–20
Geeraerts X, Bolli E, Fendt SM, Van Ginderachter JA (2017) Macrophage metabolism as therapeutic target for cancer, atherosclerosis, and obesity. Front Immunol 8:289
Viola A, Munari F, Sanchez-Rodriguez R, Scolaro T, Castegna A (2019) The metabolic signature of macrophage responses. Front Immunol 10:1462
Vitale I, Manic G, Coussens LM, Kroemer G, Galluzzi L (2019) Macrophages and metabolism in the tumor microenvironment. Cell Metab 30:36–50
Pan Y, Yu Y, Wang X, Zhang T (2020) Tumor-associated macrophages in tumor immunity. Front Immunol 11:583084
Warburg O, Wind F, Negelein E (1927) The metabolism of tumors in the body. J Gen Physiol 8:519–530
Covarrubias AJ, Aksoylar HI, Yu J, et al. (2016) Akt-mTORC1 signaling regulates Acly to integrate metabolic input to control of macrophage activation. Elife 5.
Penny HL, Sieow JL, Gun SY, et al. (2021) Targeting glycolysis in macrophages confers protection against pancreatic ductal adenocarcinoma. Int J Mol Sci 22.
Chiba S, Hisamatsu T, Suzuki H et al (2017) Glycolysis regulates LPS-induced cytokine production in M2 polarized human macrophages. Immunol Lett 183:17–23
Wu H, Yin Y, Hu X et al (2019) Effects of environmental pH on macrophage polarization and osteoimmunomodulation. ACS Biomater Sci Eng 5:5548–5557
El-Kenawi A, Gatenbee C, Robertson-Tessi M et al (2019) Acidity promotes tumour progression by altering macrophage phenotype in prostate cancer. Br J Cancer 121:556–566
Genard G, Lucas S, Michiels C (2017) Reprogramming of tumor-associated macrophages with anticancer therapies: radiotherapy versus chemo- and immunotherapies. Front Immunol 8:828
Wenes M, Shang M, Di Matteo M et al (2016) Macrophage metabolism controls tumor blood vessel morphogenesis and metastasis. Cell Metab 24:701–715
Wu H, Estrella V, Beatty M et al (2020) T-cells produce acidic niches in lymph nodes to suppress their own effector functions. Nat Commun 11:4113
Loike JD, Silverstein SC (1983) A fluorescence quenching technique using trypan blue to differentiate between attached and ingested glutaraldehyde-fixed red blood cells in phagocytosing murine macrophages. J Immunol Methods 57:373–379
Anderson M, Moshnikova A, Engelman DM, Reshetnyak YK, Andreev OA (2016) Probe for the measurement of cell surface pH in vivo and ex vivo. Proc Natl Acad Sci U S A 113:8177–8181
Li C, Levin M, Kaplan DL (2016) Bioelectric modulation of macrophage polarization. Sci Rep 6:21044
Crawford T, Moshnikova A, Roles S et al (2020) pHLIP ICG for delineation of tumors and blood flow during fluorescence-guided surgery. Sci Rep 10:18356
Wyatt LC, Lewis JS, Andreev OA, Reshetnyak YK, Engelman DM (2017) Applications of pHLIP technology for cancer imaging and therapy. Trends Biotechnol 35:653–664
Dharmaratne NU, Kaplan AR, Glazer PM (2021) Targeting the hypoxic and acidic tumor microenvironment with pH-sensitive peptides. Cells 10.
Adochite RC, Moshnikova A, Golijanin J, Andreev OA, Katenka NV, Reshetnyak YK (2016) Comparative study of tumor targeting and biodistribution of pH (low) insertion peptides (pHLIP((R)) peptides) conjugated with different fluorescent dyes. Mol Imaging Biol 18:686–696
Adochite RC, Moshnikova A, Carlin SD et al (2014) Targeting breast tumors with pH (low) insertion peptides. Mol Pharm 11:2896–2905
Moshnikova A, Moshnikova V, Andreev OA, Reshetnyak YK (2013) Antiproliferative effect of pHLIP-amanitin. Biochemistry 52:1171–1178
Reshetnyak YK, Andreev OA, Lehnert U, Engelman DM (2006) Translocation of molecules into cells by pH-dependent insertion of a transmembrane helix. Proc Natl Acad Sci U S A 103:6460–6465
Reshetnyak YK, Segala M, Andreev OA, Engelman DM (2007) A monomeric membrane peptide that lives in three worlds: in solution, attached to, and inserted across lipid bilayers. Biophys J 93:2363–2372
Andreev OA, Karabadzhak AG, Weerakkody D, Andreev GO, Engelman DM, Reshetnyak YK (2010) pH (low) insertion peptide (pHLIP) inserts across a lipid bilayer as a helix and exits by a different path. Proc Natl Acad Sci U S A 107:4081–4086
Hunt JF, Rath P, Rothschild KJ, Engelman DM (1997) Spontaneous, pH-dependent membrane insertion of a transbilayer alpha-helix. Biochemistry 36:15177–15192
Reshetnyak YK (2008) Energetics of peptide (pHLIP) binding to and folding across a lipid bilayer membrane. Chim Oggi 105:15340–15345
Kyrychenko A, Vasquez-Montes V, Ulmschneider MB, Ladokhin AS (2015) Lipid headgroups modulate membrane insertion of pHLIP peptide. Biophys J 108:791–794
Vasquez-Montes V, Gerhart J, King KE, Thevenin D, Ladokhin AS (2018) Comparison of lipid-dependent bilayer insertion of pHLIP and its P20G variant. Biochim Biophys Acta Biomembr 1860:534–543
Karabadzhak AG, Weerakkody D, Deacon J, Andreev OA, Reshetnyak YK, Engelman DM (2018) Bilayer thickness and curvature influence binding and insertion of a pHLIP peptide. Biophys J 114:2107–2115
Westerfield J, Gupta C, Scott HL et al (2019) Ions modulate key interactions between pHLIP and lipid membranes. Biophys J 117:920–929
Schlebach JP (2019) Ions at the interface: pushing the pK of pHLIP. Biophys J 117:793–794
Vasquez-Montes V, Gerhart J, Thevenin D, Ladokhin AS (2019) Divalent cations and lipid composition modulate membrane insertion and cancer-targeting action of pHLIP. J Mol Biol 431:5004–5018
Gupta C, Ren Y, Mertz B (2018) Cooperative nonbonded forces control membrane binding of the pH-low insertion peptide pHLIP. Biophys J 115:2403–2412
Brown MC, Yakubu RA, Taylor J et al (2014) Bilayer surface association of the pHLIP peptide promotes extensive backbone desolvation and helically-constrained structures. Biophys Chem 187-188:1–6
Scott HL, Heberle FA, Katsaras J, Barrera FN (2019) Phosphatidylserine asymmetry promotes the membrane insertion of a transmembrane helix. Biophys J 116:1495–1506
Li N, Yin L, Thevenin D et al (2013) Peptide targeting and imaging of damaged lung tissue in influenza-infected mice. Future Microbiol 8:257–269
Price NL, Miguel V, Ding W, et al. (2019) Genetic deficiency or pharmacological inhibition of miR-33 protects from kidney fibrosis. JCI Insight 4.
Andreev OA, Dupuy AD, Segala M et al (2007) Mechanism and uses of a membrane peptide that targets tumors and other acidic tissues in vivo. Proc Natl Acad Sci U S A 104:7893–7898
Henry KE, Chaney AM, Nagle VL, et al. (2020) Demarcation of sepsis-induced peripheral and central acidosis with pH-low insertion cyclic (pHLIC) peptide. J Nucl Med.
Sahraei M, Chaube B, Liu Y et al (2019) Suppressing miR-21 activity in tumor-associated macrophages promotes an antitumor immune response. J Clin Invest 129:5518–5536
Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S (2005) Macrophage receptors and immune recognition. Annu Rev Immunol 23:901–944
Allavena P, Chieppa M, Bianchi G et al (2010) Engagement of the mannose receptor by tumoral mucins activates an immune suppressive phenotype in human tumor-associated macrophages. Clin Dev Immunol 2010:547179
Scodeller P, Simon-Gracia L, Kopanchuk S et al (2017) Precision targeting of tumor macrophages with a CD206 binding peptide. Sci Rep 7:14655
Zhang C, Yu X, Gao L et al (2017) Noninvasive imaging of CD206-positive M2 macrophages as an early biomarker for post-chemotherapy tumor relapse and lymph node metastasis. Theranostics 7:4276–4288
Kurahara H, Takao S, Maemura K et al (2013) M2-polarized tumor-associated macrophage infiltration of regional lymph nodes is associated with nodal lymphangiogenesis and occult nodal involvement in pN0 pancreatic cancer. Pancreas 42:155–159
Wang Y, Wang J, Yang C et al (2021) A study of the correlation between M2 macrophages and lymph node metastasis of colorectal carcinoma. World J Surg Oncol 19:91
Acknowledgements
The authors are grateful for the support of Stefania Andreev for making graphics for Fig. 6, and Dr. Dhammika Weerakkody for support and maintenance of the laboratory at URI.
Funding
This work was supported by NIH grant R01 GM073857 (Y.K.R., O.A.A., and D.M.E.). Members of the URI Institutional Development Award (IDeA) Network for Biomedical Research Excellence received a support from NIH P20 GM103430.
Author information
Authors and Affiliations
Contributions
O.A.A. and Y.K.R. designed research; H.V., M.D., A.M., and T.C. performed research; H.V., M.D., A.M., T.C., and Y.R. analyzed data; and D.M.E., O.A.A., and Y.K.R. wrote the paper. All authors read, provided feedback on, and approved the manuscript for publication. H.V. and M.D. equally contributed to the work.
Corresponding author
Ethics declarations
Conflict of Interest
D.M.E., O.A.A., and Y.K.R. are founders of pHLIP, Inc., and they have shares in the company. pHLIP, Inc provided funding for the manufacturing of pHLIP ICG, the FACS analysis performed at the Charles River Discovery Labs, and the in vivo portion of the study for targeting of inflamed lungs in mice performed at Melior Discovery.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1335 kb)
Rights and permissions
About this article
Cite this article
Visca, H., DuPont, M., Moshnikova, A. et al. pHLIP Peptides Target Acidity in Activated Macrophages. Mol Imaging Biol 24, 874–885 (2022). https://doi.org/10.1007/s11307-022-01737-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-022-01737-x