Abstract
Introduction
The reliance on glycolytic metabolism is a hallmark of tumor metabolism. Excess acid and protons are produced, leading to an acidic tumor environment. Therefore, we explored the relationship between the tumor glycolytic metabolism and tissue pH by comparing 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) and hyperpolarized [1-13C]pyruvate MR spectroscopy imaging (MRSI) to chemical exchange saturation transfer (CEST) MRI measurements of tumor pH.
Methods
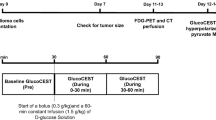
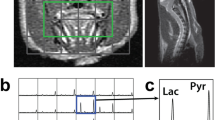
106 C6 glioma cells were implanted in the brains of male Wistar rats (N = 11) using stereotactic surgery. A 60-min PET acquisition after a bolus of FDG was performed at 11–13 days post implantation, and standardized uptake value (SUV) was calculated. CEST measurements were acquired the following day before and during constant infusion of glucose solution. Tumor intracellular pH (pHi) was evaluated using amine and amide concentration-independent detection (AACID) CEST MRI. The change of pHi (∆pHi) was calculated as the difference between pHi pre- and during glucose infusion. Rats were imaged immediately with hyperpolarized [1-13C]pyruvate MRSI. Regional maps of the ratio of Lac:Pyr were acquired. The correlations between SUV, Lac:Pyr ratio, and ∆pHi were evaluated using Pearson’s correlation.
Results
A decrease of 0.14 in pHi was found after glucose infusion in tumor region. Significant correlations between tumor glycolysis measurements of Lac:Pyr and ∆pHi within the tumor (ρ = 0.83, P = 0.01) and peritumoral region (ρ = 0.76, P = 0.028) were observed. No significant correlations were found between tumor SUV and ∆pHi within the tumor (ρ = − 0.45, P = 0.17) and peritumor regions (ρ = − 0.6, P = 0.051).
Conclusion
AACID detected the changes in pHi induced by glucose infusion. Significant correlations between tumor glycolytic measurement of Lac:Pyr and tumoral and peritumoral pHi and ∆pHi suggest the intrinsic relationship between tumor glycolytic metabolism and the tumor pH environment as well as the peritumor pH environment.






Similar content being viewed by others
References
Webb BA, Chimenti M, Jacobson MP et al (2011) Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer 11(9):671–677. https://doi.org/10.1038/nrc3110
Corbet C, Feron O (2017) Tumour acidosis: from the passenger to the driver’s seat. Nat Rev Cancer 17(10):577–593. https://doi.org/10.1038/nrc.2017.77
Estrella V, Chen T, Lloyd M et al (2013) Acidity generated by the tumor microenvironment drives local invasion. Cancer Res 73(5):1524–1535. https://doi.org/10.1158/0008-5472.CAN-12-2796
Heiden MGV, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science (80- ) 324(5930):1029–1033. https://doi.org/10.1126/science.1160809
Stubbs M, Rodrigues L, Howe FA et al (1994) Metabolic consequences of a reversed pH gradient in rat tumors. Cancer Res 54(15):4011–4016
Lim H, Albatany M, Martínez-Santiesteban F et al (2018) Longitudinal measurements of intra-and extracellular pH gradient in a rat model of glioma. Tomography 4(2):46–54. https://doi.org/10.18383/j.tom.2018.00001
Chen LQ, Howison CM, Jeffery JJ et al (2014) Evaluations of extracellular PH within in Vivo tumors using acidocest MRI. Magn Reson Med 72(5):1408–1417. https://doi.org/10.1002/mrm.25053
Volk T, JäHde E, Fortmeyer HP et al (1993) pH in human tumour xenografts: effect of intravenous administration of glucose. Br J Cancer 68(3):492–500. https://doi.org/10.1038/bjc.1993.375
Longo DL, Bartoli A, Consolino L et al (2016) In Vivo imaging of tumor metabolism and acidosis by combining PET and MRI-CEST pH imaging. Cancer Res 76(22):6463–6470. https://doi.org/10.1158/0008-5472.CAN-16-0825
Albatany M, Li A, Meakin S et al (2018) Dichloroacetate induced intracellular acidification in glioblastoma: in vivo detection using AACID-CEST MRI at 9.4 Tesla. J Neurooncol 136(2):255–262. https://doi.org/10.1007/s11060-017-2664-9
McVicar N, Li AX, Gonçalves DF et al (2014) Quantitative tissue pH measurement during cerebral ischemia using amine and amide concentration-independent detection (AACID) with MRI. J Cereb Blood Flow Metab 34(4):690–698. https://doi.org/10.1038/jcbfm.2014.12
Lim H. (2017) Tumour metabolism using hyperpolarized carbon-13 magnetic resonance spectroscopic imaging in a preclinical model of glioma. PhD Thesis
Qi Q, Fox MS, Lim H et al (2021) Multimodality in Vivo imaging of perfusion and glycolysis in a rat model of C6 glioma. Mol Imaging Biol 23:516–526. https://doi.org/10.1007/s11307-021-01585-1
Qi Q, Yeung TPC, Lee TY et al (2016) Evaluation of CT perfusion biomarkers of tumor hypoxia. PLoS ONE 11(4). https://doi.org/10.1371/journal.pone.0153569
Yeung TPC (2014) Functional imaging of malignant gliomas with CT perfusion. PhD Thesis.
Gallamini A, Zwarthoed C, Borra A (2014) Positron emission tomography (PET) in oncology. Cancers 6(4):1821–1889. https://doi.org/10.3390/cancers6041821
Silver IA, Erecinska M (1994) Extracellular glucose concentration in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J Neurosci 14(8):5068–5076. https://doi.org/10.1523/jneurosci.14-08-05068.1994
Zaiss M, Anemone A, Goerke S et al (2019) Quantification of hydroxyl exchange of D-glucose at physiological conditions for optimization of glucoCEST MRI at 3, 7 and 9.4 Tesla. NMR Biomed 32(9). https://doi.org/10.1002/nbm.4113
Zaiss M, Zu Z, Xu J et al (2015) A combined analytical solution for chemical exchange saturation transfer and semi-solid magnetization transfer. NMR Biomed 28(2):217–230. https://doi.org/10.1002/nbm.3237
Yeung TPC, Kurdi M et al (2014) CT perfusion imaging as an early biomarker of differential response to stereotactic radiosurgery in C6 rat gliomas. PLoS ONE 9(10). https://doi.org/10.1371/journal.pone.0109781
McVicar N, Li AX, Suchý M et al (2013) Simultaneous in Vivo pH and temperature mapping using a PARACEST-MRI contrast agent. Magn Reson Med 70(4):1016–1025. https://doi.org/10.1002/mrm.24539
Coman D, Huang Y, Rao JU et al (2016) Imaging the intratumoral-peritumoral extracellular pH gradient of gliomas. NMR Biomed 29(3):309–319. https://doi.org/10.1002/nbm.3466
Roesler R, Dini SA, Isolan GR (2021) Neuroinflammation and immunoregulation in glioblastoma and brain metastases: recent developments in imaging approaches. Clin Exp Immunol 206(3):1–11. https://doi.org/10.1111/CEI.13668
Golman K, Zandt R, Lerche M et al (2006) Metabolic imaging by hyperpolarized 13C magnetic resonance imaging for in Vivo tumor diagnosis. Cancer Res 66(22):10855–10860. https://doi.org/10.1158/0008-5472.CAN-06-2564
Kroemer G, Pouyssegur J (2008) Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell 13(6):472–482. https://doi.org/10.1016/J.CCR.2008.05.005
Nagano N, Sasaki H, Aoyagi M et al (1993) Invasion of experimental rat brain tumor: early morphological changes following microinjection of C6 glioma cells. Acta Neuropathol 86(2):117–125. https://doi.org/10.1007/BF00334878
Yang W, Warrington NM, Taylor SJ et al (2019) Sex differences in GBM revealed by analysis of patient imaging, transcriptome, and survival data. Sci Transl Med 11(473). https://doi.org/10.1126/SCITRANSLMED.AAO5253
Lund J, Ouwens DM, Wettergreen M et al (2019) Increased glycolysis and higher lactate production in hyperglycemic myotubes. Cells 8(9). https://doi.org/10.3390/CELLS8091101
Twarock S, Reichert C, Peter U et al (2017) Hyperglycaemia and aberrated insulin signalling stimulate tumour progression via induction of the extracellular matrix component hyaluronan. Int J cancer 141(4):791–804. https://doi.org/10.1002/IJC.30776
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qi, Q., Fox, M.S., Lim, H. et al. Glucose Infusion Induced Change in Intracellular pH and Its Relationship with Tumor Glycolysis in a C6 Rat Model of Glioblastoma. Mol Imaging Biol 25, 271–282 (2023). https://doi.org/10.1007/s11307-022-01726-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-022-01726-0