Abstract
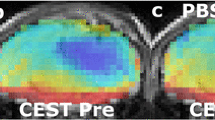
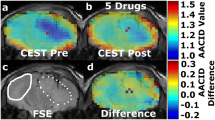
Intracellular pH (pHi) plays an important role in the maintenance of normal cell function, and is maintained within a narrow range by the activity of transporters located at the plasma membrane. Modulation of tumor pHi may influence proliferation, apoptosis, chemotherapy resistance, and thermosensitivity. Chemical exchange saturation transfer (CEST) is a novel MRI contrast mechanism that is dependent on cellular pH. Amine and amide concentration-independent detection (AACID) is a recently developed CEST contrast method that is intracellular pH (pHi) weighted. Dichloroacetate (DCA) can alter tumor pHi by inhibiting the enzyme pyruvate dehydrogenase kinase causing reduced lactate (increasing pHi), or by decreasing the expression of monocarboxylate transporters and vacuolar ATPase leading to reduced pHi. Since the net in vivo effect of DCA on pHi is difficult to predict, the purpose of this study was to quantify the magnitude of acute pHi change in glioblastoma after a single DCA injection using AACID CEST MRI. Using a 9.4T MRI scanner, CEST spectra were acquired in six mice approximately 14 days after implanting 105 U87 human glioblastoma multiforme (GBM) cells in the brain, before and after intravenous injection of DCA (dose: 200 mg/kg). Three additional mice received only phosphate buffered saline (PBS) injection and were studied as controls. Repeated measures t test was used to compare AACID changes in tumor and contralateral tissue regions of interest. One hour after DCA injection there was a significant increase in tumor AACID level by 0.04 ± 0.01 corresponding to a 0.16 decrease in pHi, and no change in AACID in contralateral tissue. Inspection of AACID maps following PBS injection showed no differences. The use of DCA to induce a tumor specific pH change detectable by AACID CEST MRI is consistent with previous studies that have shown similar effects for lonidamine and topiramate. This study demonstrates that a single dose of DCA can be used as a pharmacological challenge to induced rapid tumor intracellular acidification.



Similar content being viewed by others
Abbreviations
- GBM:
-
Glioblastoma multiforme
- pHi :
-
Intracellular pH
- pHe :
-
Extracellular pH
- PDH:
-
Pyruvate dehydrogenase
- PDK:
-
Pyruvate dehydrogenase kinase
- V-ATPase:
-
Vacuolar-type H+-ATPase
- PBS:
-
Phosphate buffered saline
- CEST:
-
Chemical exchange saturation transfer
- DCA:
-
Dichloroacetate
- RF:
-
Radiofrequency
- MTRasym :
-
Asymmetric magnetization transfer ratio
- MT:
-
Magnetization transfer
- AACID:
-
Amine and amide concentration-independent detection
- FSE:
-
Fast spin-echo
- WASSR:
-
Water saturation shift referencing
- ROI:
-
Region of interest
References
Kanu OO, Mehta A, Di C, Lin N, Bortoff K, Bigner DD, Yan H, Adamson DC (2009) Glioblastoma multiforme: a review of therapeutic targets. Expert Opin Ther Targets 13:701–718. https://doi.org/10.1517/14728220902942348
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359:492–507. https://doi.org/10.1056/NEJMra0708126
Sagiyama K, Mashimo T, Togao O, Vemireddy V, Hatanpaa KJ, Maher EA, Mickey BE, Pan E, Sherry AD, Bachoo RM, Takahashi M (2014) In vivo chemical exchange saturation transfer imaging allows early detection of a therapeutic response in glioblastoma. Proc Natl Acad Sci USA 111:4542–4547. https://doi.org/10.1073/pnas.1323855111
Holland EC (2000) Glioblastoma multiforme: the terminator. Proc Natl Acad Sci USA 97:6242–6244
Easaw JC, Mason WP, Perry J, Laperriere N, Eisenstat DD, Del Maestro R, Belanger K, Fulton D, Macdonald D (2011) Canadian recommendations for the treatment of recurrent or progressive glioblastoma multiforme. Curr Oncol 18:126–136
Zhou J, Tryggestad E, Wen Z, Lal B, Zhou T, Grossman R, Wang S, Yan K, Fu DX, Ford E, Tyler B, Blakeley J, Laterra J, van Zijl PC (2011) Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nat Med 17:130–134. https://doi.org/10.1038/nm.2268
Gerweck LE, Seetharaman K (1996) Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res 56:1194–1198
Stubbs M, Bhujwalla ZM, Tozer GM, Rodrigues LM, Maxwell RJ, Morgan R, Howe FA, Griffiths JR (1992) An assessment of 31P MRS as a method of measuring pH in rat tumours. NMR Biomed 5:351–359
Ha DH, Choi S, Oh JY, Yoon SK, Kang MJ, Kim KU (2013) Application of 31P MR spectroscopy to the brain tumors. Korean J Radiol 14:477–486. https://doi.org/10.3348/kjr.2013.14.3.477
Monika Cichocka JK, Andrzej Urbanik (2015) PH measurements of the brain using phosphorus magnetic resonance spectroscopy (31PMRS) in healthy men: comparison of two analysis methods. Pol J Radiol. https://doi.org/10.12659/PJR.895178
Oberhaensli RD, Galloway GJ, Hilton-Jones D, Bore PJ, Styles P, Rajagopalan B, Taylor DJ, Radda GK (1987) The study of human organs by phosphorus-31 topical magnetic resonance spectroscopy. Br J Radiol 60:367–373. https://doi.org/10.1259/0007-1285-60-712-367
Maintz D, Heindel W, Kugel H, Jaeger R, Lackner KJ (2002) Phosphorus-31 MR spectroscopy of normal adult human brain and brain tumours. NMR Biomed 15:18–27
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Gatenby RA, Gillies RJ (2004) Why do cancers have high aerobic glycolysis? Nat Rev Cancer 4:891–899. https://doi.org/10.1038/nrc1478
Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C, Jordan BF, Kelley MJ, Gallez B, Wahl ML, Feron O, Dewhirst MW (2008) Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest 118:3930–3942. https://doi.org/10.1172/JCI36843
Huber V, De Milito A, Harguindey S, Reshkin SJ, Wahl ML, Rauch C, Chiesi A, Pouyssegur J, Gatenby RA, Rivoltini L, Fais S (2010) Proton dynamics in cancer. J Transl Med 8:57. https://doi.org/10.1186/1479-5876-8-57
Neri D, Supuran CT (2011) Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 10:767–777. https://doi.org/10.1038/nrd3554
Webb BA, Chimenti M, Jacobson MP, Barber DL (2011) Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer 11:671–677. https://doi.org/10.1038/nrc3110
Izumi H, Torigoe T, Ishiguchi H, Uramoto H, Yoshida Y, Tanabe M, Ise T, Murakami T, Yoshida T, Nomoto M, Kohno K (2003) Cellular pH regulators: potentially promising molecular targets for cancer chemotherapy. Cancer Treat Rev 29:541–549. https://doi.org/10.1016/s0305-7372(03)00106-3
Park HJ, Makepeace CM, Lyons JC, Song CW (1996) Effect of intracellular acidity and ionomycin on apoptosis in HL-60 cells. Eur J Cancer 32A:540–546
Park HJ, Lyons JC, Ohtsubo T, Song CW (1999) Acidic environment causes apoptosis by increasing caspase activity. Br J Cancer 80:1892–1897. https://doi.org/10.1038/sj.bjc.6690617
Shrode LD, Tapper H, Grinstein S (1997) Role of intracellular pH in proliferation, transformation, and apoptosis. J Bioenerg Biomembr 29:393–399
Hubesch B, Sappey-Marinier D, Roth K, Meyerhoff DJ, Matson GB, Weiner MW (1990) P-31 MR spectroscopy of normal human brain and brain tumors. Radiology 174:401–409. https://doi.org/10.1148/radiology.174.2.2296651
McVicar N, Li AX, Goncalves DF, Bellyou M, Meakin SO, Prado MA, Bartha R (2014) Quantitative tissue pH measurement during cerebral ischemia using amine and amide concentration-independent detection (AACID) with MRI. J Cereb Blood Flow Metab 34:690–698. https://doi.org/10.1038/jcbfm.2014.12
Zong X, Wang P, Kim SG, Jin T (2014) Sensitivity and source of amine-proton exchange and amide-proton transfer magnetic resonance imaging in cerebral ischemia. Magn Reson Med 71:118–132. https://doi.org/10.1002/mrm.24639
Zhou JY, Payen JF, Wilson DA, Traystman RJ, van Zijl PCM (2003) Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med 9:1085–1090. https://doi.org/10.1038/nm907
Zhou J LB, Wilson DA, Laterra J, van Zijl PC (2003) Amide proton transfer (APT) contrast for imaging of brain tumors. Magn Reson Med 50 https://doi.org/10.1002/mrm.10651
Murray RKGD (2003) Membranes: structure and function. McGraw-Hill Companies, Inc, New York, pp 415–433
McVicar N, Li AX, Meakin SO, Bartha R (2015) Imaging chemical exchange saturation transfer (CEST) effects following tumor-selective acidification using lonidamine. NMR Biomed 28:566–575. https://doi.org/10.1002/nbm.3287
Marathe K, McVicar N, Li A, Bellyou M, Meakin S, Bartha R (2016) Topiramate induces acute intracellular acidification in glioblastoma. J Neurooncol 130:465–472. https://doi.org/10.1007/s11060-016-2258-y
Michelakis ED, Webster L, Mackey JR (2008) Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br J Cancer 99:989–994. https://doi.org/10.1038/sj.bjc.6604554
Ishiguro T, Ishiguro M, Ishiguro R, Iwai S (2012) Cotreatment with dichloroacetate and omeprazole exhibits a synergistic antiproliferative effect on malignant tumors. Oncol Lett 3:726–728. https://doi.org/10.3892/ol.2012.552
Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Bonnet S, Harry G, Hashimoto K, Porter CJ, Andrade MA, Thebaud B, Michelakis ED (2007) A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 11:37–51. https://doi.org/10.1016/j.ccr.2006.10.020
Dai Y, Xiong X, Huang G, Liu J, Sheng S, Wang H, Qin W (2014) Dichloroacetate enhances adriamycin-induced hepatoma cell toxicity in vitro and in vivo by increasing reactive oxygen species levels. PLoS ONE 9:e92962. https://doi.org/10.1371/journal.pone.0092962
Kankotia S, Stacpoole PW (2014) Dichloroacetate and cancer: new home for an orphan drug? Biochim Biophys Acta 1846:617–629. https://doi.org/10.1016/j.bbcan.2014.08.005
Cairns RA, Papandreou I, Sutphin PD, Denko NC (2007) Metabolic targeting of hypoxia and HIF1 in solid tumors can enhance cytotoxic chemotherapy. Proc Natl Acad Sci USA 104:9445–9450. https://doi.org/10.1073/pnas.0611662104
Kumar A, Kant S, Singh SM (2013) Antitumor and chemosensitizing action of dichloroacetate implicates modulation of tumor microenvironment: a role of reorganized glucose metabolism, cell survival regulation and macrophage differentiation. Toxicol Appl Pharmacol 273:196–208. https://doi.org/10.1016/j.taap.2013.09.005
Park JM, Recht LD, Josan S, Merchant M, Jang T, Yen YF, Hurd RE, Spielman DM, Mayer D (2013) Metabolic response of glioma to dichloroacetate measured in vivo by hyperpolarized (13)C magnetic resonance spectroscopic imaging. Neuro Oncol 15:433–441. https://doi.org/10.1093/neuonc/nos319
Sanchez WY, McGee SL, Connor T, Mottram B, Wilkinson A, Whitehead JP, Vuckovic S, Catley L (2013) Dichloroacetate inhibits aerobic glycolysis in multiple myeloma cells and increases sensitivity to bortezomib. Br J Cancer 108:1624–1633. https://doi.org/10.1038/bjc.2013.120
Li AX, Suchy M, Li C, Gati JS, Meakin S, Hudson RH, Menon RS, Bartha R (2011) In vivo detection of MRI-PARACEST agents in mouse brain tumors at 9.4 T. Magn Reson Med 66:67–72. https://doi.org/10.1002/mrm.22772
Kim M, Gillen J, Landman BA, Zhou J, van Zijl PC (2009) Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn Reson Med 61:1441–1450. https://doi.org/10.1002/mrm.21873
Michelakis ED, Sutendra G, Dromparis P, Webster L, Haromy A, Niven E, Maguire C, Gammer TL, Mackey JR, Fulton D, Abdulkarim B, McMurtry MS, Petruk KC (2010) Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med 2:31ra34. https://doi.org/10.1126/scitranslmed.3000677
Pecqueur C, Oliver L, Oizel K, Lalier L, Vallette FM (2013) Targeting metabolism to induce cell death in cancer cells and cancer stem cells. Int J Cell Biol 2013:805975 https://doi.org/10.1155/2013/805975
Desmond KL, Moosvi F, Stanisz GJ (2014) Mapping of amide, amine, and aliphatic peaks in the CEST spectra of murine xenografts at 7 T. Magn Reson Med 71:1841–1853. https://doi.org/10.1002/mrm.24822
Feldman SC, Chu D, Schulder M, Pawar R, Barry M, Cho E, Liu W (2009) The blood oxygen level-dependent functional MR imaging signal can be used to identify brain tumors and distinguish them from normal tissue. Am J Neuroradiol 30:389–395. https://doi.org/10.3174/ajnr.A1326
Acknowledgements
Funding for this study was provided by the Ontario Institute of Cancer Research (OICR) Smarter Imaging Program (Grant Number 00807) with support from Brain Canada. The authors wish to thank Miranda Bellyou for assistance with animal preparation and handling. Thanks to Misan University-Ministry of Higher Education and Scientific Research, Iraq.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All applicable national and institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Albatany, M., Li, A., Meakin, S. et al. Dichloroacetate induced intracellular acidification in glioblastoma: in vivo detection using AACID-CEST MRI at 9.4 Tesla. J Neurooncol 136, 255–262 (2018). https://doi.org/10.1007/s11060-017-2664-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2664-9