Abstract
Purpose
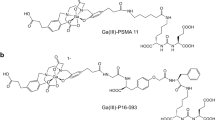
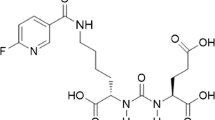
Prostate-specific membrane antigen (PSMA) is a promising molecular target for imaging of prostate adenocarcinoma. 68Ga-P16-093, a small molecule PSMA ligand, previously showed equivalent diagnostic performance compared to 68Ga-PSMA-11 PET/CT in a pilot study of prostate cancer patients with biochemical recurrence (BCR). We performed a pilot study for further characterization of 68Ga-P16-093 including comparison to conventional imaging.
Procedures
Patients were enrolled into two cohorts. The biodistribution cohort included 8 treated prostate cancer patients without recurrence, who underwent 6 whole body PET/CT scans with urine sampling for dosimetry using OLINDA/EXM. The dynamic cohort included 15 patients with BCR and 2 patients with primary prostate cancer. Two patients with renal cell carcinoma were also enrolled for exploratory use. A dynamic PET/CT was followed by 2 whole body scans for imaging protocol optimization based on bootstrapped replicates. 68Ga-P16-093 PET/CT was compared for diagnostic performance against available 18F-fluciclovine PET/CT, 99mTc-MDP scintigraphy, diagnostic CT, and MRI.
Results
68Ga-P16-093 deposited similar effective dose (0.024 mSv/MBq) and lower urinary bladder dose (0.064 mSv/MBq) compared to 68Ga-PSMA-11. The kidneys were the critical organ (0.290 mSv/MBq). While higher injected activities were preferable, lower injected activities at 74–111 MBq (2–3 mCi) yielded 80% retention in signal-to-noise ratio. The optimal injection-to-scan interval was 60 min, with acceptable delay up to 90 min. 68Ga-P16-093 PET/CT showed superior diagnostic performance over conventional imaging with overall patient-level lesion detection rate of 71%, leading to a change in management in 42% of the patients.
Conclusions
Based on its favorable imaging characteristics and diagnostic performance in prostate cancer, 68Ga-P16-093 PET/CT merits further investigation in larger clinical studies.







Similar content being viewed by others
References
Davis MI, Bennett MJ, Thomas LM, Bjorkman PJ (2005) Crystal structure of prostate-specific membrane antigen, a tumor marker and peptidase. Proc Natl Acad Sci U S A 102:5981–5986
Zippel C, Ronski SC, Bohnet-Joschko S, Giesel FL, Kopka K (2020) Current status of PSMA- radiotracers for prostate cancer: data analysis of prospective trials listed on ClinicalTrials.gov. Pharmaceuticals (Basel) 13:12
Rhee H, Blazak J, Tham CM et al (2016) Pilot study: use of gallium-68 PSMA PET for detection of metastatic lesions in patients with renal tumour. EJNMMI Res 6:76
Hofman MS, Hicks RJ, Maurer T, Eiber M (2018) Prostate-specific membrane antigen PET: clinical utility in prostate cancer, normal patterns, pearls, and pitfalls. Radiographics 38:200–217
Han S, Woo S, Kim YJ, Suh CH (2018) Impact of (68)Ga-PSMA PET on the management of patients with prostate cancer: a systematic review and meta-analysis. Eur Urol 74:179–190
Pienta KJ, Gorin MA, Rowe SP et al (2021) A phase 2/3 prospective multicenter study of the diagnostic accuracy of prostate specific membrane antigen PET/CT with (18)F-DCFPyL in prostate cancer patients (OSPREY). J Urol 206:52–61
Fendler WP, Calais J, Eiber M et al (2019) Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol 5:856–863
Eiber M, Maurer T, Souvatzoglou M et al (2015) Evaluation of hybrid (6)(8)Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med 56:668–674
U.S. Food and Drug Administration. Drug trials snapshot: Ga 68 PSMA-11. www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshot-ga-68-psma-11. Accessed 6 Mar 2021
Morris MJ, Rowe SP, Gorin MA et al (2021) Diagnostic performance of (18)F-DCFPyL-PET/CT in men with biochemically recurrent prostate cancer: results from the CONDOR Phase III, multicenter study. Clin Cancer Res 27:3674–3682
Zha Z, Ploessl K, Choi SR, Wu Z, Zhu L, Kung HF (2018) Synthesis and evaluation of a novel urea-based (68)Ga-complex for imaging PSMA binding in tumor. Nucl Med Biol 59:36–47
Green MA, Hutchins GD, Bahler CD et al (2020) [(68)Ga]Ga-P16-093 as a PSMA-targeted PET radiopharmaceutical for detection of cancer: initial evaluation and comparison with [(68)Ga]Ga-PSMA-11 in prostate cancer patients presenting with biochemical recurrence. Mol Imaging Biol 22:752–763
Kolthammer JA, Su KH, Grover A, Narayanan M, Jordan DW, Muzic RF (2014) Performance evaluation of the Ingenuity TF PET/CT scanner with a focus on high count-rate conditions. Phys Med Biol 59:3843–3859
Stabin MG, Sparks RB, Crowe E (2005) OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med 46:1023–1027
Herrmann K, Bluemel C, Weineisen M et al (2015) Biodistribution and radiation dosimetry for a probe targeting prostate-specific membrane antigen for imaging and therapy. J Nucl Med 56:855–861
Haynor DR, Woods SD (1989) Resampling estimates of precision in emission tomography. IEEE Trans Med Imaging 8:337–343
Dahlbom M (2002) Estimation of image noise in PET using the bootstrap method. IEEE Trans Nucl Sci 49:2062–2066
Demirci E, Sahin OE, Ocak M, Akovali B, Nematyazar J, Kabasakal L (2016) Normal distribution pattern and physiological variants of 68Ga-PSMA-11 PET/CT imaging. Nucl Med Commun 37:1169–1179
Shetty D, Patel D, Le K, Bui C, Mansberg R (2018) Pitfalls in gallium-68 PSMA PET/CT interpretation-a pictorial review. Tomography 4:182–193
Ceci F, Oprea-Lager DE, Emmett L et al (2021) E-PSMA: the EANM standardized reporting guidelines v1.0 for PSMA-PET. Eur J Nucl Med Mol Imaging 48:1626–1638
Pfob CH, Ziegler S, Graner FP et al (2016) Biodistribution and radiation dosimetry of (68)Ga-PSMA HBED CC-a PSMA specific probe for PET imaging of prostate cancer. Eur J Nucl Med Mol Imaging 43:1962–1970
Afshar-Oromieh A, Hetzheim H, Kratochwil C et al (2015) The theranostic PSMA ligand PSMA-617 in the diagnosis of prostate cancer by PET/CT: biodistribution in humans, radiation dosimetry, and first evaluation of tumor lesions. J Nucl Med 56:1697–1705
Afshar-Oromieh A, Hetzheim H, Kubler W et al (2016) Radiation dosimetry of (68)Ga-PSMA-11 (HBED-CC) and preliminary evaluation of optimal imaging timing. Eur J Nucl Med Mol Imaging 43:1611–1620
U.S. Food and Drug Administration. Gallium Ga 68 PSMA-11 injection, for intravenous use [prescribing information]. Revised December 2020. www.accessdata.fda.gov/drugsatfda_docs/label/2020/212642s000lbl.pdf. Accessed August 27, 2021
U.S. Food and Drug Administration. PYLARIFY® (piflufolastat F 18) injection, for intravenous use [prescribing information]. www.accessdata.fda.gov/drugsatfda_docs/label/2021/214793s000lbl.pdf. Accessed January 2, 2022
Burke LM, Bashir MR, Neville AM, Nelson RC, Jaffe TA (2014) Current opinions on medical radiation: a survey of oncologists regarding radiation exposure and dose reduction in oncology patients. J Am Coll Radiol 11:490–495
National Comprehensive Cancer Network. Clinical practice guidelines in oncology: prostate cancer (Version 2.2021). www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed August 27, 2021
Boellaard R, Delgado-Bolton R, Oyen WJ et al (2015) FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 42:328–354
Tan N, Oyoyo U, Bavadian N et al (2020) PSMA-targeted radiotracers versus (18)F fluciclovine for the detection of prostate cancer biochemical recurrence after definitive therapy: a systematic review and meta-analysis. Radiology 296:44–55
Yuminaga Y, Rothe C, Kam J et al (2021) (68)Ga-PSMA PET/CT versus CT and bone scan for investigation of PSA failure post radical prostatectomy. Asian J Urol 8:170–175
Hope TA, Goodman JZ, Allen IE, Calais J, Fendler WP, Carroll PR (2019) Metaanalysis of (68)Ga-PSMA-11 PET accuracy for the detection of prostate cancer validated by histopathology. J Nucl Med 60:786–793
Petersen LJ, Zacho HD (2020) PSMA PET for primary lymph node staging of intermediate and high-risk prostate cancer: an expedited systematic review. Cancer Imaging 20:10
Hope TA, Eiber M, Armstrong WR et al (2021) Diagnostic accuracy of 68Ga-PSMA-11 PET for pelvic nodal metastasis detection prior to radical prostatectomy and pelvic lymph node dissection: a multicenter prospective phase 3 imaging trial. JAMA Oncol 7:1635–1642
Hofman MS, Lawrentschuk N, Francis RJ et al (2020) Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet 395:1208–1216
Lopci E, Saita A, Lazzeri M et al (2018) (68)Ga-PSMA positron emission tomography/computerized tomography for primary diagnosis of prostate cancer in men with contraindications to or negative multiparametric magnetic resonance imaging: a prospective observational study. J Urol 200:95–103
Raveenthiran S, Esler R, Yaxley J, Kyle S (2019) The use of (68)Ga-PET/CT PSMA in the staging of primary and suspected recurrent renal cell carcinoma. Eur J Nucl Med Mol Imaging 46:2280–2288
Sawicki LM, Buchbender C, Boos J et al (2017) Diagnostic potential of PET/CT using a (68)Ga-labelled prostate-specific membrane antigen ligand in whole-body staging of renal cell carcinoma: initial experience. Eur J Nucl Med Mol Imaging 44:102–107
Gao J, Xu Q, Fu Y et al (2021) Comprehensive evaluation of (68)Ga-PSMA-11 PET/CT parameters for discriminating pathological characteristics in primary clear-cell renal cell carcinoma. Eur J Nucl Med Mol Imaging 48:561–569
Gühne F, Seifert P, Theis B, Steinert M, Freesmeyer M, Drescher R (2021) PSMA-PET/CT in patients with recurrent clear cell renal cell carcinoma: histopathological correlations of imaging findings. Diagnostics (Basel) 11:1142
Fani M, André JP, Maecke HR (2008) 68Ga-PET: a powerful generator-based alternative to cyclotron-based PET radiopharmaceuticals. Contrast Media Mol Imaging 3:67–77
Hong H, Wang G, Ploessl K et al (2021) Kit-based preparation of [(68)Ga]Ga-P16-093 (PSMA-093) using different commercial (68)Ge/(68)Ga generators. Nucl Med Biol 106–107:1–9
Funding
Funding support for the work was received from Five Eleven Pharma. Five Eleven Pharma’s development and synthesis of 68Ga-P16-093 were supported by NIH/NCI SBIR grants 1R44CA233140-01 and 1R43CA217425-01.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
The University of Pennsylvania Institutional Review Board approved the study. All the procedures performed involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all the individual participants included in the study.
Conflict of Interest
David Alexoff: CEO of Five Eleven Pharma Inc.
Hank F. Kung: Founder and Chairman of the board at Five Eleven Pharma Inc.
Daniel A. Pryma: Research Consultant, Five Eleven Pharma Inc.; Research Consultant, Progenics Pharmaceuticals Inc.; Research Consultant, Actinium Pharmaceuticals Inc.; Research Consultant, Ipsen; Research Grant, Siemens AG; Research Grant, Five Eleven Pharma Inc.; Research Grant, Progenics Pharmaceuticals Inc.; Clinical Trial Funding, Nordic Nanovector ASA.
The other authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, H., Scheuermann, J.S., Young, A.J. et al. Preliminary Evaluation of 68Ga-P16-093, a PET Radiotracer Targeting Prostate-Specific Membrane Antigen in Prostate Cancer. Mol Imaging Biol 24, 710–720 (2022). https://doi.org/10.1007/s11307-022-01720-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-022-01720-6