Abstract
Purpose
This is a first-in-human study to evaluate the radiation dosimetry of a new prostate-specific membrane antigen (PSMA)-targeted radiopharmaceutical, [18F]AlF-P16-093, and also initial investigation of its ability to detect PSMA-positive tumors using PET scans in a cohort of prostate cancer (PCa) patients.
Methods
The [18F]AlF-P16-093 was automatically synthesized with a GE TRACERlab. A total of 23 patients with histopathologically proven PCa were prospectively enrolled. Dosimetry and biodistribution study investigations were carried out on a subset of six (6) PCa patients, involving multiple time-point scanning. The mean absorbed doses were estimated with PMOD and OLINDA software.
Results
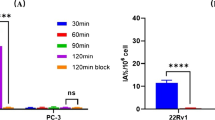
[18F]AlF-P16-093 was successfully synthesized, and radiochemical purity was > 95%, and average labeling yield was 36.5 ± 8.3% (decay correction, n = 12). The highest tracer uptake was observed in the kidneys, spleen, and liver, contributing to an effective dose of 16.8 ± 1.3 μSv/MBq, which was ~ 30% lower than that of [68Ga]Ga-P16-093. All subjects tolerated the PET examination well, and no reportable side-effects were observed. The PSMA-positive tumors displayed rapid uptake, and they were all detectable within 10 min, and no additional lesions were observed in the following multi-time points scanning. Each patient had at least one detectable tumor lesion, and a total of 356 tumor lesions were observed, including intraprostatic, lymph node metastases, bone metastases, and other soft tissue metastases.
Conclusions
We report herein a streamlined method for high yield synthesis of [18F]AlF-P16-093. Preliminary study in PCa patients has demonstrated its safety and acceptable radiation dosimetry. The initial diagnostic study indicated that [18F]AlF-P16-093 PET/CT is efficacious and potentially useful for a widespread application in the diagnosis of PCa patients.





Similar content being viewed by others
Data availability
Datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. Ca Cancer J Clin. 2023;73:17–48. https://doi.org/10.3322/caac.21763.
Simon NI, Parker C, Hope TA, Paller CJ. Best approaches and updates for prostate cancer biochemical recurrence. Am Soc Clin Oncol Educ Book. 2022;42:1–8. https://doi.org/10.1200/edbk_351033.
Panebianco V, Villeirs G, Weinreb JC, Turkbey BI, Margolis DJ, Richenberg J, et al. Prostate magnetic resonance imaging for local recurrence reporting (PI-RR): International Consensus -based guidelines on multiparametric magnetic resonance imaging for prostate cancer recurrence after radiation therapy and radical prostatectomy. Eur Urology Oncol. 2021;4:868–76. https://doi.org/10.1016/j.euo.2021.01.003.
Hope TA, Eiber M, Armstrong WR, Juarez R, Murthy V, Lawhn-Heath C, et al. Diagnostic accuracy of 68Ga-PSMA-11 PET for pelvic nodal metastasis detection prior to radical prostatectomy and pelvic lymph node dissection: a multicenter prospective phase 3 imaging trial. JAMA Oncol. 2021;7:1635–42. https://doi.org/10.1001/jamaoncol.2021.3771.
Fendler WP, Eiber M, Beheshti M, Bomanji J, Calais J, Ceci F, et al. PSMA PET/CT: joint EANM procedure guideline/SNMMI procedure standard for prostate cancer imaging 2.0. Eur J Nucl Med Mol Imaging. 2023;50:1466-86. https://doi.org/10.1007/s00259-022-06089-w
Farolfi A, Calderoni L, Mattana F, Mei R, Telo S, Fanti S, et al. Current and emerging clinical applications of PSMA PET diagnostic imaging for prostate cancer. J Nucl Med. 2021;62:596–604. https://doi.org/10.2967/jnumed.120.257238.
Neels OC, Kopka K, Liolios C, Afshar-Oromieh A. Radiolabeled PSMA inhibitors. Cancers. 2021;13. https://doi.org/10.3390/cancers13246255.
Jochumsen MR, Bouchelouche K. PSMA PET/CT for primary staging of prostate cancer-an updated overview. Semin Nucl Med. 2023Jul 22;S0001–2998(23):00055–7. https://doi.org/10.1053/j.semnuclmed.2023.07.001.
Carlucci G, Ippisch R, Slavik R, Mishoe A, Blecha J, Zhu S. (68)Ga-PSMA-11 NDA approval: a novel and successful academic partnership. J Nucl Med. 2021;62:149–55. https://doi.org/10.2967/jnumed.120.260455.
Ulaner GA, Thomsen B, Bassett J, Torrey R, Cox C, Lin K, et al. 18F-DCFPyL PET/CT for initially diagnosed and biochemically recurrent prostate cancer: prospective trial with pathologic confirmation. Radiology. 2022;305:419–28. https://doi.org/10.1148/radiol.220218.
Kuppermann D, Calais J, Marks LS. Imaging prostate cancer: clinical utility of prostate-specific membrane antigen. J Urol. 2022;207:769–78. https://doi.org/10.1097/JU.0000000000002457.
Voter AF, Werner RA, Pienta KJ, Gorin MA, Pomper MG, Solnes LB, et al. Piflufolastat F-18 (18F-DCFPyL) for PSMA PET imaging in prostate cancer. Expert Rev Anticancer Ther. 2022;22:681–94. https://doi.org/10.1055/a-1659-0010.
Eiber M, Kroenke M, Wurzer A, Ulbrich L, Jooss L, Maurer T, et al. (18)F-rhPSMA-7 PET for the detection of biochemical recurrence of prostate cancer after radical prostatectomy. J Nucl Med. 2020;61:696–701. https://doi.org/10.2967/jnumed.119.234914.
Barna S, Haug AR, Hartenbach M, Rasul S, Grubmüller B, Kramer G, et al. Dose calculations and dose-effect relationships in 177Lu-PSMA I&T radionuclide therapy for metastatic castration-resistant prostate cancer. Clin Nucl Med. 2020;45:661–7. https://doi.org/10.1097/rlu.0000000000003157.
Houshmand S, Lawhn-Heath C, Behr S. PSMA PET imaging in the diagnosis and management of prostate cancer. Abdom Radiol (NY). 2023:1-14. https://doi.org/10.1007/s00261-023-04002-z.
Jani AB, Ravizzini GC, Gartrell BA, Siegel BA, Twardowski P, Saltzstein D, et al. Diagnostic performance and safety of 18F-rhPSMA-7.3 positron emission tomography in men with suspected prostate cancer recurrence: results from a phase 3, prospective, multicenter study (SPOTLIGHT). J Urol. 2023:10-1097. https://doi.org/10.1097/JU.0000000000003493
Surasi DS, Eiber M, Maurer T, Preston MA, Helfand BT, Josephson D, et al. Diagnostic performance and safety of positron emission tomography with 18F-rhPSMA-7.3 in patients with newly diagnosed unfavourable intermediate-to very-high-risk prostate cancer: results from a phase 3, prospective, multicentre study (LIGHTHOUSE). Eur Urol. 2023. https://doi.org/10.1016/j.eururo.2023.06.018.
Debnath S, Zhou N, McLaughlin M, Rice S, Pillai AK, Hao G, et al. PSMA-targeting imaging and theranostic agents-current status and future perspective. Int J Mol Sci. 2022;23:1158–77. https://doi.org/10.3390/ijms23031158.
Alati S, Singh R, Pomper MG, Rowe SP, Banerjee SR. Preclinical development in radiopharmaceutical therapy for prostate cancer. Semin Nucl Med. 2023;0:1-12. https://doi.org/10.1053/j.semnuclmed.2023.06.007.
Archibald SJ, Allott L. The aluminium-[(18)F]fluoride revolution: simple radiochemistry with a big impact for radiolabelled biomolecules. EJNMMI Radiopharm Chem. 2021;6:30. https://doi.org/10.1186/s41181-021-00141-0.
De Man K, Van Laeken N, Schelfhout V, Fendler WP, Lambert B, Kersemans K, et al. (18)F-PSMA-11 versus (68)Ga-PSMA-11 positron emission tomography/computed tomography for staging and biochemical recurrence of prostate cancer: a prospective double-blind randomised cross-over trial. Eur Urol. 2022;82:501–9. https://doi.org/10.1016/j.eururo.2022.05.010.
Piron S, De Man K, Van Laeken N, D’Asseler Y, Bacher K, Kersemans K, et al. Radiation dosimetry and biodistribution of 18F-PSMA-11 for PET imaging of prostate cancer. J Nucl Med. 2019;60:1736–42. https://doi.org/10.2967/jnumed.118.225250.
dos Santos G, Taroco MR, Giglio J, Savio E, Alonso O. Al18F-PSMA-HBED-CC as a novel tracer for the evaluation of prostate cancer patients with biochemical relapse: intraindividual comparison with 68Ga-PSMA-HBED-CC. J Nucl Med. 2020;61:1268.
Bowling GC, Dimitrakoff JD. PSMA PET in prostate cancer—a biomarker or a surrogate end point? JAMA oncology. 2022;8:1. https://doi.org/10.1001/jamaoncol.2021.7991.
Zha Z, Ploessl K, Choi SR, Wu Z, Zhu L, Kung HF. Synthesis and evaluation of a novel urea-based 68Ga-complex for imaging PSMA binding in tumor. Nucl Med Biol. 2018;59:36–47. https://doi.org/10.1016/j.nucmedbio.2017.12.007.
Wang G, Hong H, Zang J, Liu Q, Jiang Y, Fan X, et al. Head-to-head comparison of [68Ga]Ga-P16-093 and [68Ga]Ga-PSMA-617 in dynamic PET/CT evaluation of the same group of recurrent prostate cancer patients. Eur J Nucl Med Mol Imaging. 2022;49:1052–62. https://doi.org/10.1007/s00259-021-05539-1.
Wang G, Li L, Zhu M, Zang J, Wang J, Wang R, et al. A prospective head-to-head comparison of [(68)Ga]Ga-P16-093 and [(68)Ga]Ga-PSMA-11 PET/CT in patients with primary prostate cancer. Eur J Nucl Med Mol Imaging. 2023;50:3126–36. https://doi.org/10.1007/s00259-023-06283-4.
Wang G, Li L, Zang J, Hong H, Zhu L, Kung HF, et al. Head-to-head comparison of 68Ga-P16-093 and 68Ga-PSMA-617 PET/CT in patients with primary prostate cancer: a pilot studY. Clin Nucl Med. 2023;48:289–95. https://doi.org/10.1097/rlu.0000000000004566.
Lee H, Scheuermann JS, Young AJ, Doot RK, Daube-Witherspoon ME, Schubert EK, et al. Preliminary evaluation of 68Ga-P16-093, a PET radiotracer targeting prostate-specific membrane antigen in prostate cancer. Mol Imaging Biol. 2022;26:1–11. https://doi.org/10.1007/s11307-022-01720-6.
Green MA, Hutchins GD, Bahler CD, Tann M, Mathias CJ, Territo W, et al. [68Ga]Ga-P16-093 as a PSMA-targeted PET radiopharmaceutical for detection of cancer: initial evaluation and comparison with [68Ga]Ga-PSMA-11 in prostate cancer patients presenting with biochemical recurrence. Mol Imaging Biol. 2020;22:752–63. https://doi.org/10.1007/s11307-019-01421-7.
Zha Z, Choi SR, Ploessl K, Alexoff D, Zhao R, Zhu L, et al. Radiolabeling optimization and preclinical evaluation of the new PSMA imaging agent [(18)F]AlF-P16-093. Bioconjug Chem. 2021;32:1017–26. https://doi.org/10.1021/acs.bioconjchem.1c00177.
Valentin J. Basic anatomical and physiological data for use in radiological protection: reference values: ICRP Publication 89. Annals of the ICRP. 2002;32:1-277. https://doi.org/10.1016/S0146-6453(03)00002-2.
Ringheim A, Campos Neto GC, Anazodo U, Cui L, da Cunha ML, Vitor T, et al. Kinetic modeling of (68)Ga-PSMA-11 and validation of simplified methods for quantification in primary prostate cancer patients. EJNMMI Res. 2020;10:12. https://doi.org/10.1186/s13550-020-0594-6.
Gafita A, Wang H, Robertson A, Armstrong WR, Zaum R, Weber M, et al. Tumor sink effect in (68)Ga-PSMA-11 PET: myth or reality? J Nucl Med. 2022;63:226–32. https://doi.org/10.2967/jnumed.121.261906.
Piron S, De Man K, Van Laeken N, D’Asseler Y, Bacher K, Kersemans K, et al. Radiation dosimetry and biodistribution of (18)F-PSMA-11 for PET imaging of prostate cancer. J Nucl Med. 2019;60:1736–42. https://doi.org/10.2967/jnumed.118.225250.
Alberts I, Sachpekidis C, Dijkstra L, Prenosil G, Gourni E, Boxler S, et al. The role of additional late PSMA-ligand PET/CT in the differentiation between lymph node metastases and ganglia. Eur J Nucl Med Mol Imaging. 2020;47:642–51. https://doi.org/10.1007/s00259-019-04552-9.
Hohberg M, Kobe C, Täger P, Hammes J, Schmidt M, Dietlein F, et al. Combined early and late [68Ga]PSMA-HBED-CC PET scans improve lesion detectability in biochemical recurrence of prostate cancer with low PSA levels. Mol Imaging Biol. 2019;21:558–66. https://doi.org/10.1007/s11307-018-1263-2.
Dietlein F, Kobe C, Neubauer S, Schmidt M, Stockter S, Fischer T, et al. PSA-stratified performance of (18)F- and (68)Ga-PSMA PET in patients with biochemical recurrence of prostate cancer. J Nucl Med. 2017;58:947–52. https://doi.org/10.2967/jnumed.116.185538.
Zacho HD, Ravn S, Afshar-Oromieh A, Fledelius J, Ejlersen JA, Petersen LJ. Added value of (68)Ga-PSMA PET/CT for the detection of bone metastases in patients with newly diagnosed prostate cancer and a previous (99m)Tc bone scintigraphy. EJNMMI Res. 2020;10:31. https://doi.org/10.1186/s13550-020-00618-0.
Acknowledgements
The authors thank Dr. William Eckelman and Dr. Guochang Wang for advice and suggestions in preparation of this manuscript.
Funding
This study was supported by Guangdong regional joint fund (2022A1515110941); Guangzhou basic and applied basic research (2023A04J1196), Science and Technology Program of Guangzhou (202201020558) and enhancing scientific research in Guangzhou Medical University.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Ruiyue Zhao, Miao Ke, Jie Lv, Shaoyu Liu, Yuheng Liu, Jing Zhang, Lifu Xu, Di Gu, Mingzhao Li, Chao Cai, Yongda Liu, Guohua Zeng, David Alexoff, Karl Ploessl, Lin Zhu, Hank F. Kung, and Xinlu Wang. The first draft of the manuscript was written by Ruiyue Zhao, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
This study was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (ES-2023-141), and it was conducted in accordance with the principles of the Declaration of Helsinki.
Consent to participate
Informed consent was obtained from all participants included in the study
Conflict of interest
David Alexoff, Karl Ploessl, and Hank F. Kung are employees of Five Eleven Pharma, and Hank Kung is also the found and board of the company, which holds the patent rights for [18F]AlF-P16-093 and related technology. Other authors have no conflicts of interest or relevant financial activities to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, R., Ke, M., Lv, J. et al. First-in-human study of PSMA-targeting agent, [18F]AlF-P16-093: dosimetry and initial evaluation in prostate cancer patients. Eur J Nucl Med Mol Imaging 51, 1753–1762 (2024). https://doi.org/10.1007/s00259-024-06596-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-024-06596-y