Abstract
Purpose
Current checkpoint inhibitor immunotherapy strategies in glioblastoma are challenged by mechanisms of resistance including an immunosuppressive tumor microenvironment. T cell immunoglobulin domain and mucin domain 3 (TIM3) is a late-phase checkpoint receptor traditionally associated with T cell exhaustion. We apply fluorescent imaging techniques to explore feasibility of in vivo visualization of the immune state in a glioblastoma mouse model.
Procedures
TIM3 monoclonal antibody was conjugated to a near-infrared fluorescent dye, IRDye-800CW (800CW). The TIM3 experimental conjugate and isotype control were assessed for specificity with immunofluorescent staining and flow cytometry in murine cell lines (GL261 glioma and RAW264.7 macrophages). C57BL/6 mice with orthotopically implanted GL261 cells were imaged in vivo over 4 days after intravenous TIM3-800CW injection to assess tumor-specific uptake. Cell-specific uptake was then assessed on histologic sections.
Results
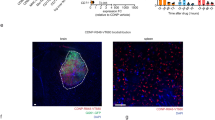
The experimental TIM3-800CW, but not its isotype control, bound to RAW264.7 macrophages in vitro. Specificity to RAW264.7 macrophages and not GL261 tumor cells was quantitatively confirmed with the corresponding clone of TIM3 on flow cytometry. In vivo fluorescence imaging of the 800CW signal was localized to the intracranial tumor and significantly higher for the TIM3-800CW cohort, relative to non-targeting isotype control, immediately after tail vein injection and for up to 48 h after injection. Resected organs of tumor bearing mice showed significantly higher uptake in the liver and spleen. TIM3-800CW was seen to co-stain with CD3 (13%), CD11b (29%), and CD206 (26%).
Conclusions
We propose fluorescent imaging of immune cell imaging as a potential strategy for monitoring and localizing immunologically relevant foci in the setting of brain tumors. Alternative markers and target validation will further clarify the temporal relationship of immunosuppressive effector cells throughout glioma resistance.




Similar content being viewed by others
References
Lim M, Xia Y, Bettegowda C, Weller M (2018) Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol 15:422–442
McGranahan T, Therkelsen KE, Ahmad S, Nagpal S (2019) Current state of immunotherapy for treatment of glioblastoma. Curr Treat Options Oncol 20:24
Jackson CM, Choi J, Lim M (2019) Mechanisms of immunotherapy resistance: lessons from glioblastoma. Nat Immunol 20:1100–1109
Anderson AC, Joller N, Kuchroo VK (2016) Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity 44:989–1004
Kim JE, Patel MA, Mangraviti A et al (2017) Combination therapy with anti-PD-1, anti-TIM-3, and focal radiation results in regression of murine gliomas. Clin Cancer Res 23:124–136
Mazurek M, Kulesza B, Stoma F, Osuchowski J, Mańdziuk S, Rola R (2020) Characteristics of fluorescent intraoperative dyes helpful in gross total resection of high-grade gliomas-a systematic review. Diagnostics (Basel) 10(12):1100. https://doi.org/10.3390/diagnostics10121100
Zhou Q, van den Berg N, Rosenthal E et al (2021) EGFR-targeted intraoperative fluorescence imaging detects high-grade glioma with panitumumab-IRDye800 in a phase 1 clinical trial. Thernostics 11(15):7130–7143. https://doi.org/10.7150/thno.60582
Du Y, Sun T, Liang X et al (2017) Improved resection and prolonged overall survival with PD-1-IRDye800CW fluorescence probe-guided surgery and PD-1 adjuvant immunotherapy in 4T1 mouse model. Int J Nanomedicine 12:8337–8351
Li L, Du Y, Chen X, Tian J (2018) Fluorescence molecular imaging and tomography of matrix metalloproteinase-activatable near-infrared fluorescence probe and image-guided orthotopic glioma resection. Mol Imaging Biol 20:930–939
Wissler HL, Ehlerding EB, Lyu Z et al (2019) Site-specific immuno-PET tracer to image PD-L1. Mol Pharm 16:2028–2036
Nishio N, van den Berg NS, van Keulen S et al (2019) Optical molecular imaging can differentiate metastatic from benign lymph nodes in head and neck cancer. Nat Commun 10:1–10
Yecies D, Liba O, SoRelle ED et al (2019) Speckle modulation enables high-resolution wide-field human brain tumor margin detection and in vivo murine neuroimaging. Sci Rep 9:1–9
Prabhakar U, Maeda H, Jain RK et al (2013) Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res 73(8):2412–2417. https://doi.org/10.1158/0008-5472.CAN-12-4561
Zhou Q, Vega Leonel JCM, Santoso MR et al (2021) Molecular imaging of a fluorescent antibody against epidermal growth factor receptor detects high-grade glioma. Sci Rep 11:5710
Reardon DA, Brandes AA, Omuro A et al (2020) Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol 6:1003–1010
Anderson AC, Anderson DE, Bregoli L et al (2007) Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science 318:1141–1143
Cole KE, Ly QP, Hollingsworth MA et al (2020) Comparative phenotypes of peripheral blood and spleen cells from cancer patients. Int Immunopharmacol 85:106655
Velásquez-Lopera MM, Correa LA, García LF (2008) Human spleen contains different subsets of dendritic cells and regulatory T lymphocytes. Clin Exp Immunol 154:107–114
Koks CA, De Vleeschouwer S, Graf N, Van Gool SW (2015) Immune suppression during oncolytic virotherapy for high-grade glioma; yes or no? J Cancer 6:203–217
Jang BS, Kim IA (2020) A radiosensitivity gene signature and PD-L1 status predict clinical outcome of patients with glioblastoma multiforme in the cancer genome atlas dataset. Cancer Res Treat 52:530–542
Wei J, Montalvo-Ortiz W, Yu L et al (2021) Sequence of αPD-1 relative to local tumor irradiation determines the induction of abscopal antitumor immune responses. Sci Immunol 6(58):eabg0117. https://doi.org/10.1126/sciimmunol.abg0117
Williamson CW, Sherer MV, Zamarin D et al (2021) Immunotherapy and radiation therapy sequencing: state of the data on timing, efficacy, and safety. Cancer 127:1553–1567
Xia W, Zhu J, Tang Y et al (2020) PD-L1 inhibitor regulates the miR-33a-5p/PTEN signaling pathway and can be targeted to sensitize glioblastomas to radiation. Front Oncol 10:821
Li G, Wang Z, Zhang C et al (2017) Molecular and clinical characterization of TIM-3 in glioma through 1,024 samples. Oncoimmunology 6:e1328339
Li X, Wang B, Gu L et al (2018) Tim-3 expression predicts the abnormal innate immune status and poor prognosis of glioma patients. Clin Chim Acta 476:178–184
Funding
MZ receives research funding from the National Institutes of Health (5T32CA009695-27, MPI).
Author information
Authors and Affiliations
Contributions
Conception and design: MZ, QZ, CTC, GL, ML, SSG, HED.
Acquisition of data/materials: MZ, QZ, CH, WW, CTC, SSG, HED.
Analysis of data: MZ, QZ, CH, CTC.
Drafting of manuscript: MZ.
Critical revision: QZ, CH, CTC, WW, GL, ML, SSG, HED.
Final approval: MZ, QZ, CH, CTC, WW, GL, ML, SSG, HED.
Agreement of accountability: MZ, QZ, CH, CTC, WW, GL, ML, SSG, HED.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, M., Zhou, Q., Huang, C. et al. In Vivo Evaluation of Near-Infrared Fluorescent Probe for TIM3 Targeting in Mouse Glioma. Mol Imaging Biol 24, 280–287 (2022). https://doi.org/10.1007/s11307-021-01667-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-021-01667-0