Abstract
Purpose
Molecular imaging of tumor HER2 expression may allow patient selection for HER2-targeted therapies. Our aim was to introduce hexahistidine (His6) peptides into pertuzumab Fab to enable labeling with the [99mTc(CO)3(H2O)3]+ complex and study these radioimmunoconjugates for microSPECT/CT imaging of HER2-positive tumor xenografts in mice.
Procedures
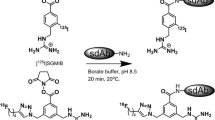
Fab were produced by papain digestion of pertuzumab and reacted with sulfo-SMCC for conjugation to His6-containing peptides (CGYGGHHHHHH). His6-peptide conjugation was measured by a radiometric assay. His6-pertuzumab Fab were labeled at 0.4–1.0 MBq/μg with [99mTc(CO)3(H2O)3]+ for 1 h at 37 °C. HER2 immunoreactivity was assessed in a direct (saturation) binding assay using HER2-overexpressing SK-BR-3 human breast cancer (BC) cells. MicroSPECT/CT and biodistribution studies were performed in NOD/SCID mice with HER2-positive s.c. SK-OV-3 human ovarian cancer, or MDA-MB-361 or MDA-MB-231 human BC xenografts at 4 or 24 h post i.v. injection of [99mTc]His6-pertuzumab Fab (29–49 MBq, 70 μg). The specificity of tumor uptake was assessed by comparison to irrelevant [99mTc]Fab 3913 in SK-OV-3 tumor-bearing mice.
Results
SDS-PAGE analysis demonstrated cleavage of pertuzumab to produce Fab, which eluted as a single peak with a retention time of 13.8 min on SE-HPLC. Fab were conjugated to 2.1 ± 0.5 His6 peptides and labeled with [99mTc(CO)3(H2O)3]+ to a radiochemical purity of 92–97 % at 0.4–0.8 MBq/μg. [99mTc]His6-pertuzumab Fab exhibited saturable and specific binding to SK-BR-3 cells with a KD = 51.3 ± 5.2 × 10−9 M and Bmax = 3.5 ± 0.1 × 106 receptors/cell. SK-OV-3 tumors were imaged at 4 and 24 h p.i [99mTc]His6-pertuzumab Fab. Tumor uptake at 24 h p.i. was 4.1 ± 0.6 %ID/g, which was 13-fold significantly greater than [99mTc]Fab 3913 (0.3 ± 0.0 %ID/g; P < 0.01). MicroSPECT/CT imaged HER2-overexpressing MDA-MB-361 tumors but not MDA-MB-231 tumors with low HER2 expression. Tumor uptake was 5.2-fold significantly greater at 24 h p.i. in MDA-MB-361 than MDA-MB-231 tumors (3.2 ± 0.1 %ID/g vs. 0.8 ± 0.1 %ID/g; P < 0.05).
Conclusions
MicroSPECT/CT with [99mTc]His6-pertuzumab Fab imaged tumors in NOD/SCID mice that exhibited intermediate or high HER2 expression, but not tumors with low HER2. [99mTc]His6-pertuzumab Fab is promising for SPECT imaging of tumor HER2 expression.




Similar content being viewed by others
References
Yan M, Schwaederle M, Arguello D, Millis SZ, Gatalica Z, Kurzrock R (2015) HER2 expression status in diverse cancers: review of results from 37,992 patients. Cancer Metastasis Rev 34:157–164
Oh DY, Bang YJ (2020) HER2-targeted therapies - a role beyond breast cancer. Nat Rev Clin Oncol 17:33–48
Slamon DJ, Clark GM, Wong SG, Levin W, Ullrich A, McGuire W (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177–182
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Láng I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Rüschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD, Herceptin Adjuvant (HERA) Trial Study Team (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353:1659–1672
Swain SM, Kim SB, Cortés J, Ro J, Semiglazov V, Campone M, Cruelos E, Ferrero JM, Schneeweiss A, Knott A, Clark E, Ross G, Benyunes MC, Baselga J (2013) Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 14:461–471
Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, Andre F, Iwata H, Ito Y, Tsurutani J, Sohn J, Denduluri N, Perrin C, Aogi K, Tokunaga E, Im SA, Lee KS, Hurvitz SA, Cortes J, Lee C, Chen S, Zhang L, Shahidi J, Yver A, Krop I, DESTINY-Breast01 Investigators (2020) Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med 382:610–621
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, Jenkins RB, Press MF, Spears PA, Vance GH, Viale G, McShane LM, Dowsett M (2018) Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med 142:1364–1382
Rossi S, Basso M, Strippoli A, Dadduzio V, Cerchiaro E, Barile R, D'Argento E, Cassano A, Schinzari G, Barone C (2015) Hormone receptor status and HER2 expression in primary breast cancer compared with synchronous axillary metastases or recurrent metastatic disease. Clin Breast Cancer 15:307–312
Vu T, Claret FX (2012) Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front Oncol 2:62
Niikura N, Liu J, Hayashi N, Mittendorf EA, Gong Y, Palla SL, Tokuda Y, Gonzalez-Angulo AM, Hortobagyi GN, Ueno NT (2012) Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J Clin Oncol 30:593–599
Ulaner GA, Lyashchenko SK, Riedl C et al (2017) First-in-human HER2-argeted imaging using 89Zr-pertuzumab PT/CT: dosimetry and clinical application in patients with breast cancer. J Nucl Med 59:900–906
Gebhart G, Lamberts LE, Wimana Z, Garcia C, Emonts P, Ameye L, Stroobants S, Huizing M, Aftimos P, Tol J, Oyen WJG, Vugts DJ, Hoekstra OS, Schröder CP, Menke-van der Houven van Oordt CW, Guiot T, Brouwers AH, Awada A, de Vries EGE, Flamen P (2016) Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): the ZEPHIR trial. Ann Onco 27:619–624
Ulaner GA, Offin M, Scaltriti M et al (2019) 89Zr-trastuzumab PET/CT for prediction of response to HER2-targeted therapy in patients with HER2 mutant lung cancer: an exploratory phase 2 trial. J Nucl Med 60(Suppl 1):144
Gebhart G, Flamen P, De Vries EG, Jhaveri K, Wimana Z (2016) Imaging diagnostic and therapeutic targets: human epidermal growth factor receptor 2. J Nucl Med 57:81S–88S
Sinclair A, Morrison A, Young C, Pyke L. (2018) The Canadian medical imaging inventory, 2017. Ottawa: CADTH
Ku A, Chan C, Aghevlian S, Cai Z, Cescon D, Bratman SV, Ailles L, Hedley DW, Reilly RM (2019) MicroSPECT/CT imaging of cell-line and patient-derived EGFR-positive tumor xenografts in mice with panitumumab fab modified with hexahistidine peptides to enable labeling with 99mTc(I) tricarbonyl complex. Mol Pharm 16:3559–3568
Waibel R, Alberto R, Willuda J, Finnern R, Schibli R, Stichelberger A, Egli A, Abram U, Mach JP, Plückthun A, Schubiger PA (1999) Stable one-step technetium-99m labeling of his-tagged recombinant proteins with a novel Tc(I)-carbonyl complex. Nat Biotechnol 17:897–901
McLarty K, Cornelissen B, Scollard DA, Done SJ, Chun K, Reilly RM (2009) Associations between the uptake of 111In-DTPA-trastuzumab, HER2 density and response to trastuzumab (Herceptin) in athymic mice bearing subcutaneous human tumour xenografts. Eur J Nucl Med Mol Imaging 36:81–93
Orlova A, Tolmachev V, Pehrson R, Lindborg M, Tran T, Sandström M, Nilsson FY, Wennborg A, Abrahmsén L, Feldwisch J (2007) Synthetic affibody molecules: a novel class of affinity ligands for molecular imaging of HER2-expressing malignant tumors. Cancer Res 67:2178–2186
Gill SC, von Hippel PH (1989) Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem 182:319–326
Chen P, Wang J, Hope K, Jin L, Dick J, Cameron R, Brandwein J, Minden M, Reilly RM (2006) Nuclear localizing sequences (NLS) promote nuclear translocation and enhance the radiotoxicity of the anti-CD33 monoclonal antibody HuM195 labeled with 111In in human myeloid leukemia cells. J Nucl Med 47:827–836
McLarty K, Cornelissen B, Cai Z, Scollard DA, Costantini DL, Done SJ, Reilly RM (2009) Micro-SPECT/CT with 111In-DTPA-pertuzumab sensitively detects trastuzumab-mediated HER2 downregulation and tumor response in athymic mice bearing MDA-MB-361 human breast cancer xenografts. J Nucl Med 50:1340–1348
Lam K, Chan C, Reilly RM (2017) Development and preclinical studies of 64Cu-NOTA-pertuzumab F(ab')2 for imaging changes in tumor HER2 expression associated with response to trastuzumab by PET/CT. MAbs 9:154–164
Aghevlian S, Lu Y, Winnik MA, Hedley DW, Reilly RM (2018) Panitumumab modified with metal-chelating polymers (MCP) complexed to 111In and 177Lu – an EGFR-targeted theranostic for pancreatic cancer. Mol Pharm 15:1150–1159
Boyle AJ, Cao PJ, Hedley DW, Sidhu SS, Winnik MA, Reilly RM (2015) MicroPET/CT imaging of patient-derived pancreatic cancer xenografts implanted subcutaneously or orthotopically in NOD-scid mice using 64Cu-NOTA-panitumumab F(ab')2 fragments. Nucl Med Biol 42:71–77
Jain RK (1990) Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer Res 50(Suppl 3):814s–819s
Oude Munnink TH, Dijkers E, Lub-de Hooge M, Kosterink J, Brouwers A, de Jong J, van Dongen G, de Vries E (2009) HER-2-PET imaging with 89Zr-trastuzumab in metastatic breast cancer patients. J Clin Oncol 27(Suppl 15):1045
Acknowledgments
The authors thank Deborah Scollard and Teesha Komal at the STTARR Innovation Centre for technical support. The authors also thank Dr. Sachdev Sidhu at the Toronto Recombinant Antibody Centre at the University of Toronto for providing Fab 3913.
Funding
This study was supported by a grant from the Canadian Cancer Society to RMR (Grant #704660). VF and NA received scholarships from the STARS21 strategic training program in radiation research supported by the Terry Fox Foundation. VF is supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) by the Polymer Nanoparticles for Drug Delivery (PoND) Training Program, MDS Nordion Graduate Scholarship in Radiopharmaceutical Sciences (OTSS), and William Knapp Buckley Award. VF and AK received scholarships from the Centre for Pharmaceutical Oncology at the University of Toronto. NA was supported by a fellowship with the Precision Medicine Initiative (PRiME) at the University of Toronto. AK is supported by the Queen Elizabeth II Graduate Scholarship in Science and Technology (QEII-GSST). Pertuzumab was provided by Genentech Inc. (South San Francisco, CA, USA) through a Materials Transfer Agreement with the University of Toronto.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 199 kb)
Rights and permissions
About this article
Cite this article
Facca, V.J., Al-saden, N., Ku, A. et al. Imaging of HER2-Positive Tumors in NOD/SCID Mice with Pertuzumab Fab-Hexahistidine Peptide Immunoconjugates Labeled with [99mTc]-(I)-Tricarbonyl Complex. Mol Imaging Biol 23, 495–504 (2021). https://doi.org/10.1007/s11307-020-01571-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-020-01571-z