Abstract
Purpose
We aimed to develop a gallium-68 (Ga-68)-labeled single-chain variable fragment (scFv) targeting the human epidermal growth factor receptor 2 (HER2) to rapidly and noninvasively evaluate the status of HER2 expression.
Procedures
Anti-HER2 scFv was labeled with Ga-68 by using deferoxamine (Df) as a bifunctional chelate. Biodistribution of [68Ga]Df-anti-HER2 scFv was examined with tumor-bearing mice and positron emission tomography (PET) imaging was performed. The changes in HER2 expression after anti-HER2 therapy were monitored by PET imaging.
Results
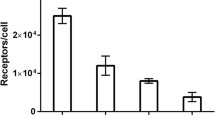
[68Ga]Df-anti-HER2 scFv was obtained with high radiochemical yield after only a 5-min reaction at room temperature. The probe showed high accumulation in HER2-positive xenografts and the intratumoral distribution of radioactivity coincided with HER2-positive regions. Furthermore, [68Ga]Df-anti-HER2 scFv helped visualize HER2-positive xenografts and monitor the changes in HER2 expression after anti-HER2 therapy.
Conclusion
[68Ga]Df-anti-HER2 scFv could be a promising probe to evaluate HER2 status by in vivo PET imaging, unless trastuzumab is prescribed as part of the therapy.




Similar content being viewed by others
References
Chua TC, Merrett ND (2012) Clinicopathologic factors associated with HER2-positive gastric cancer and its impact on survival outcomes—a systematic review. Int J Cancer 130:2845–2856
Stern HM (2012) Improving treatment of HER2-positive cancers: opportunities and challenges. Sci Transl Med 4:127rv122
McAlpine JN, Wiegand KC, Vang R et al (2009) HER2 overexpression and amplification is present in a subset of ovarian mucinous carcinomas and can be targeted with trastuzumab therapy. BMC Cancer 9:433
Nielsen DL, Kumler I, Palshof JA, Andersson M (2013) Efficacy of HER2-targeted therapy in metastatic breast cancer. Monoclonal antibodies and tyrosine kinase inhibitors. Breast 22:1–12
Sauter G, Lee J, Bartlett JM et al (2009) Guidelines for human epidermal growth factor receptor 2 testing: biologic and methodologic considerations. J Clin Oncol 27:1323–1333
Warneke VS, Behrens HM, Boger C et al (2013) Her2/neu testing in gastric cancer: evaluating the risk of sampling errors. Ann Oncol 24:725–733
Fabi A, Di Benedetto A, Metro G et al (2011) HER2 protein and gene variation between primary and metastatic breast cancer: significance and impact on patient care. Clin Cancer Res 17:2055–2064
Capala J, Bouchelouche K (2010) Molecular imaging of HER2-positive breast cancer: a step toward an individualized 'image and treat' strategy. Curr Opin Oncol 22:559–566
Tamura K, Kurihara H, Yonemori K et al (2013) 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J Nucl Med 54:1869–1875
Dijkers EC, Oude Munnink TH, Kosterink JG et al (2010) Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther 87:586–592
Smith-Jones PM, Solit DB, Akhurst T et al (2004) Imaging the pharmacodynamics of HER2 degradation in response to Hsp90 inhibitors. Nat Biotechnol 22:701–706
Reddy S, Shaller CC, Doss M et al (2011) Evaluation of the anti-HER2 C6.5 diabody as a PET radiotracer to monitor HER2 status and predict response to trastuzumab treatment. Clin Cancer Res 17:1509–1520
Olafsen T, Kenanova VE, Sundaresan G et al (2005) Optimizing radiolabeled engineered anti-p185HER2 antibody fragments for in vivo imaging. Cancer Res 65:5907–5916
Decristoforo C, Pickett RD, Verbruggen A (2012) Feasibility and availability of 68Ga-labelled peptides. Eur J Nucl Med Mol Imaging 39(Suppl 1):S31–S40
Jagoda EM, Lang L, Bhadrasetty V et al (2012) Immuno-PET of the hepatocyte growth factor receptor Met using the 1-armed antibody onartuzumab. J Nucl Med 53:1592–1600
Vosjan MJ, Perk LR, Roovers RC et al (2011) Facile labelling of an anti-epidermal growth factor receptor Nanobody with 68Ga via a novel bifunctional desferal chelate for immuno-PET. Eur J Nucl Med Mol Imaging 38:753–763
Boros E, Ferreira CL, Yapp DT et al (2012) RGD conjugates of the H2dedpa scaffold: synthesis, labeling and imaging with 68Ga. Nucl Med Biol 39:785–794
Shimizu Y, Temma T, Hara I et al (2014) Micelle-based activatable probe for in vivo near-infrared optical imaging of cancer biomolecules. Nanomedicine 10:187–195
Nakase I, Konishi Y, Ueda M et al (2012) Accumulation of arginine-rich cell-penetrating peptides in tumors and the potential for anticancer drug delivery in vivo. J Control Release 159:181–188
Ueda M, Ogawa K, Miyano A et al (2013) Development of an oxygen-sensitive degradable Peptide probe for the imaging of hypoxia-inducible factor-1-active regions in tumors. Mol Imaging Biol 15:713–721
Ueda M, Fukushima T, Ogawa K et al (2014) Synthesis and evaluation of a radioiodinated peptide probe targeting alphavbeta6 integrin for the detection of pancreatic ductal adenocarcinoma. Biochem Biophys Res Commun 445:661–666
Kudo T, Ueda M, Konishi H et al (2011) PET imaging of hypoxia-inducible factor-1-active tumor cells with pretargeted oxygen-dependent degradable streptavidin and a novel 18 F-labeled biotin derivative. Mol Imaging Biol 13:1003–1010
Ono M, Cheng Y, Kimura H et al (2013) Development of novel 123I-labeled pyridyl benzofuran derivatives for SPECT imaging of beta-amyloid plaques in Alzheimer’s disease. PLoS One 8:e74104
Adams GP, Schier R, McCall AM et al (2001) High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules. Cancer Res 61:4750–4755
Garcia-Carbonero R, Carnero A, Paz-Ares L (2013) Inhibition of HSP90 molecular chaperones: moving into the clinic. Lancet Oncol 14:e358–e369
Baum RP, Prasad V, Muller D et al (2010) Molecular imaging of HER2-expressing malignant tumors in breast cancer patients using synthetic 111In- or 68Ga-labeled affibody molecules. J Nucl Med 51:892–897
Tolmachev V, Velikyan I, Sandstrom M, Orlova A (2010) A HER2-binding affibody molecule labelled with 68Ga for PET imaging: direct in vivo comparison with the 111In-labelled analogue. Eur J Nucl Med Mol Imaging 37:1356–1367
Ren G, Zhang R, Liu Z et al (2009) A 2-helix small protein labeled with 68Ga for PET imaging of HER2 expression. J Nucl Med 50:1492–1499
Honarvar H, Jokilaakso N, Andersson K et al (2013) Evaluation of backbone-cyclized HER2-binding 2-helix affibody molecule for in vivo molecular imaging. Nucl Med Biol 40:378–386
Kondo N, Temma T, Shimizu Y et al (2013) Miniaturized antibodies for imaging membrane type-1 matrix metalloproteinase in cancers. Cancer Sci 104:495–501
Adams GP, McCartney JE, Tai MS et al (1993) Highly specific in vivo tumor targeting by monovalent and divalent forms of 741 F8 anti-c-erbB-2 single-chain Fv. Cancer Res 53:4026–4034
El Hage Chahine JM, Hemadi M, Ha-Duong NT (2012) Uptake and release of metal ions by transferrin and interaction with receptor 1. Biochim Biophys Acta 1820:334–347
Pinto AC, Ades F, de Azambuja E, Piccart-Gebhart M (2013) Trastuzumab for patients with HER2 positive breast cancer: delivery, duration and combination therapies. Breast 22(Suppl 2):S152–S155
Huang D, Lu N, Fan Q et al (2013) HER2 status in gastric and gastroesophageal junction cancer assessed by local and central laboratories: Chinese results of the HER-EAGLE Study. PLoS One 8:e80290
Ono N, Yamazaki T, Nakanishi Y et al (2012) Preclinical antitumor activity of the novel heat shock protein 90 inhibitor CH5164840 against human epidermal growth factor receptor 2 (HER2)-overexpressing cancers. Cancer Sci 103:342–349
Trepel J, Mollapour M, Giaccone G, Neckers L (2010) Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer 10:537–549
Chandarlapaty S, Scaltriti M, Angelini P et al (2010) Inhibitors of HSP90 block p95-HER2 signaling in Trastuzumab-resistant tumors and suppress their growth. Oncogene 29:325–334
Jhaveri K, Miller K, Rosen L et al (2012) A phase I dose-escalation trial of trastuzumab and alvespimycin hydrochloride (KOS-1022; 17 DMAG) in the treatment of advanced solid tumors. Clin Cancer Res 18:5090–5098
Kramer-Marek G, Gijsen M, Kiesewetter DO et al (2012) Potential of PET to predict the response to trastuzumab treatment in an ErbB2-positive human xenograft tumor model. J Nucl Med 53:629–637
Acknowledgments
The authors would like to thank FUJIFILM RI Pharma Co., Ltd. for providing gallium-67 chloride and supporting the use of 68Ge/68Ga generator. The authors would like to thank Canon Inc. for providing anti-HER2 scFv. The authors are grateful to Central Research Laboratory, Okayama University Medical School for MALDI-TOF-MS analyses, and Okayama Medical Inovation Center for the assistance of image analyses. This work was supported in part by the Research and Development Project on Molecular Probes for Detection of Biological Features on Cancer of the New Energy and Industrial Technology Development Organization (NEDO), Japan, and a Grant-in-Aid for Scientific Research (KAKENHI No. 23000005) from the Japan Society for the Promotion of Science.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ueda, M., Hisada, H., Temma, T. et al. Gallium-68-Labeled Anti-HER2 Single-Chain Fv Fragment: Development and In Vivo Monitoring of HER2 Expression. Mol Imaging Biol 17, 102–110 (2015). https://doi.org/10.1007/s11307-014-0769-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-014-0769-5