Abstract
Purpose
We aimed to use 68Ga-citrate, a labeled product of gallium (iron analog), combined with positron emission tomography/computed tomography (PET/CT) to non-invasively evaluate the potential of the iron-responsive product dihydroartemisinin (DHA) in the treatment of rheumatoid arthritis.
Procedures
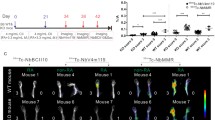
From the establishment of chicken II collagen-induced arthritis (CIA) rat model over 40 days, 20 rats with one-to-one corresponding arthritis index (AI) scores were randomly divided into two groups. One group received oral DHA (at a dose of 1.5 ml/(kg day), containing 20 mg DHA per 1 ml) for 15 days; the other group received stroke-physiological saline solution (SSS, 1.5 ml/(kg day) for 15 days. 68Ga-citrate micro-PET/CT imaging was performed on day 0 (D0), day 5 (D5), day 10 (D10), and day 15 (D15) of oral administration. After data reconstruction, the cross-sectional length “d” of the ankle joint of each rat was measured on the transverse CT, and the SUVmax of the ankle joint and muscle background was measured for statistical analysis. After micro-PET/CT collection, the ankle joint tissue was observed by HE staining.
Results
The ankle joint swelling in the DHA group was significantly suppressed, but the SSS group showed no significant suppression. 68Ga-citrate micro-PET/CT imaging results and microscope observation confirmed this finding. Statistical analysis indicated that the time tendency of AI score (Binteraction = 0.495, P < 0.001) and T/NT (Binteraction = 1.345, P < 0.001) were discrepant between DHA and SSS groups. The AI score and T/NT of the DHA group gradually increased with time, while the SSS group score gradually decreased. Furthermore, the Spearman correlation coefficient was used to describe the relationship between “d” and T/NT, which was positively correlated (r = 0.855, P < 0.001).
Conclusions
This study showed that the anti-inflammatory effect of the iron-responsive product DHA in arthritis can be monitored by an iron-like radioactive tracer (68Ga-citrate).



Similar content being viewed by others
References
Chaudhari K, Rizvi S, Syed BA (2016) Rheumatoid arthritis: current and future trends. Nat Rev Drug Discov 15(5):305–306
Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, Williams B, Gabriel S, Lassere M, Johns N, Buchbinder R, Woolf A, March L (2014) The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 73(7):1316–1322
Brown PM, Isaacs JD (2014) Rheumatoid arthritis: from palliation to remission in two decades. Clin Med (Lond) 14(Suppl 6):s50–s55
Cheong DHJ, Tan DWS, Wong FWS, Tran T (2020) Anti-malarial drug, artemisinin and its derivatives for the treatment of respiratory diseases. Pharmacol Res 158:104901
Zhang Y, He W, Du Y et al (2020) Dimeric artesunate phospholipid-conjugated liposomes as promising anti-inflammatory therapy for rheumatoid arthritis. Int J Pharm 579:119178
Mu X, Wang C (2018) Artemisinins-a promising new treatment for systemic lupus erythematosus: a descriptive review. Curr Rheumatol Rep 20:55
Khakzad MR, Ganji A, Ariabod V, Farahani I (2017) (2017) Artemisinin therapeutic efficacy in the experimental model of multiple sclerosis. Immunopharmacol Immunotoxicol 39:348–353
Isaacs JD, Harari O, Kobold U, Lee JS, Bernasconi C (2013) Effect of tocilizumab on haematological markers implicates interleukin-6 signalling in the anaemia of rheumatoid arthritis. Arthritis Res Ther 15:R204
Petzer V, Theurl I, Weiss G (2018) Established and emerging concepts to treat imbalances of iron homeostasis in inflammatory diseases. Pharmaceuticals (Basel) 11
Guillen C, McInnes IB, Kruger H et al (1998) Iron, lactoferrin and iron regulatory protein activity in the synovium; relative importance of iron loading and the inflammatory response. Ann Rheum Dis 57:309–314
Gopalakrishnan AM, Kumar N (2015) Antimalarial action of artesunate involves DNA damage mediated by reactive oxygen species. Antimicrob Agents Chemother 59:317–325
Haynes RK, Cheu KW, N'Da D et al (2013) Considerations on the mechanism of action of artemisinin antimalarials: part 1--the 'carbon radical' and 'heme' hypotheses. Infect Disord Drug Targets 13:217–277
Cao P, Leng D, Li Y, Zhang Z, Liu L, Li X (2016) Progress on anti-tumor molecular mechanisms of dihydroartemisinin. Zhejiang Da Xue Xue Bao Yi Xue Ban 45:501–507
Shen Y, Zhang B, Su Y, Badshah SA, Wang X, Li X, Xue Y, Xie L, Wang Z, Yang Z, Zhang G, Shang P (2020) Iron promotes dihydroartemisinin cytotoxicity via ROS production and blockade of autophagic flux via lysosomal damage in osteosarcoma. Front Pharmacol 11:444
Tang T, Xu W, Ma J, Wang H, Cui Z, Jiang T, Li C (2019) Inhibitory mechanisms of DHA/CQ on pH and iron homeostasis of erythrocytic stage growth of plasmodium falciparum. Molecules 24
Chen GQ, Benthani FA, Wu J, Liang D, Bian ZX, Jiang X (2020) Artemisinin compounds sensitize cancer cells to ferroptosis by regulating iron homeostasis. Cell Death Differ 27:242–254
Fan M, Li Y, Yao C, Liu X, Liu J, Yu B (2018) DC32, a Dihydroartemisinin derivative, ameliorates collagen-induced arthritis through an Nrf2-p62-Keap1 feedback loop. Front Immunol 9:2762
Xu T (2020) Chen Y (2020) research Progress of [68Ga]citrate PET's utility in infection and inflammation imaging: a review. Mol Imaging Biol 22(1):22–32
Mari AC, Behr SC, Seo Y et al (2017) Imaging hepatocellular carcinoma with (68)Ga-citrate PET: first clinical experience. Mol Imaging 16:1536012117723256
Behr SC, Aggarwal R, Seo Y, Aparici CM, Chang E, Gao KT, Tao DH, Small EJ, Evans MJ (2016) A feasibility study showing [(68)Ga]citrate PET detects prostate cancer. Mol Imaging Biol 18:946–951
Wang Z, Cai L, Xu T et al (2019) Comparative evaluation of 68Ga-citrate PET/CT and 18F-FDG PET/CT in the diagnosis of type II collagen-induced arthritis in rats. Contrast Media Mol Imaging 2019:2353658
Zhao YG, Wang Y, Guo Z, Gu AD, Dan HC, Baldwin AS, Hao W, Wan YY (2012) Dihydroartemisinin ameliorates inflammatory disease by its reciprocal effects on Th and regulatory T cell function via modulating the mammalian target of rapamycin pathway. J Immunol 189(9):4417–4425
Fan M, Li Y, Yao C, Liu X, Liu X, Liu J (2018) Dihydroartemisinin derivative DC32 attenuates collagen-induced arthritis in mice by restoring the Treg/Th17 balance and inhibiting synovitis through down-regulation of IL-6. Int Immunopharmacol 65:233–243
Funding
This work was funded by the Nuclear Medicine and Molecular Imaging Key Laboratory of Sichuan Province.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All studies were approved by the Ethics Committee of Southwestern Medical University, and all applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zi Wang and Yu Hou contribute equally to this work.
Rights and permissions
About this article
Cite this article
Wang, Z., Hou, Y., Cai, L. et al. The Evaluation of 68Ga-Citrate PET/CT Imaging for Dihydroartemisinin in the Treatment of Rheumatoid Arthritis. Mol Imaging Biol 23, 30–37 (2021). https://doi.org/10.1007/s11307-020-01534-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-020-01534-4