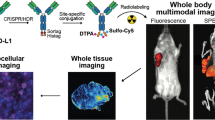
Abstract
Purpose
The swell of new and diverse radiotracers to predict or monitor tumor response to cancer immunotherapies invites the opportunity for comparative studies to identify optimal platforms. To probe the significance of antibody format on image quality for PD-L1 imaging, we developed and studied the biodistribution of a library of antibodies based on the anti-PD-L1 IgG1 clone C4.
Procedure
A C4 minibody and scFv were cloned, expressed, and characterized. The antibodies were functionalized with desferrioxamine and radiolabeled with Zr-89 to enable a rigorous comparison with prior data collected using 89Zr-labeled C4 IgG1. The biodistribution of the radiotracers was evaluated in C57Bl6/J or nu/nu mice bearing B16F10 or H1975 tumors, respectively, which are models that represent high and low tumor autonomous PD-L1 expression.
Results
The tumor uptake of the 89Zr-C4 minibody was higher than 89Zr-C4 scFv and equivalent to previous data collected using 89Zr-C4 IgG1. However, the peak tumors to normal tissue ratios were generally higher for 89Zr-C4 scFv compared with 89Zr-C4 minibody and 89Zr-IgG1. Moreover, an exploratory study showed that the rapid clearance of 89Zr-C4 scFv enabled detection of endogenous PD-L1 on a genetically engineered and orthotopic model of hepatocellular carcinoma.
Conclusion
In summary, these data support the use of low molecular weight constructs for PD-L1 imaging, especially for tumor types that manifest in abdominal organs that are obstructed by the clearance of high molecular weight radioligands.




Similar content being viewed by others
References
Schumacher TN, Kesmir C, van Buuren MM (2015) Biomarkers in cancer immunotherapy. Cancer Cell 27:12–14
Patel SP, Kurzrock R (2015) PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther 14:847–856
Havel JJ, Chowell D, Chan TA (2019) The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer 19:133–150
Ehlerding EB, England CG, McNeel DG, Cai W (2016) Molecular imaging of immunotherapy targets in cancer. J Nucl Med 57:1487–1492
Krekorian M, Fruhwirth GO, Srinivas M, Figdor CG, Heskamp S, Witney TH, Aarntzen EHJG (2019) Imaging of T-cells and their responses during anti-cancer immunotherapy. Theranostics 9:7924–7947
Davis AA, Patel VG (2019) The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer 7:278
Bensch F, van der Veen EL, Lub-de Hooge MN, Jorritsma-Smit A, Boellaard R, Kok IC, Oosting SF, Schröder CP, Hiltermann TJN, van der Wekken AJ, Groen HJM, Kwee TC, Elias SG, Gietema JA, Bohorquez SS, de Crespigny A, Williams SP, Mancao C, Brouwers AH, Fine BM, de Vries EGE (2018) (89)Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med 24:1852–1858
Niemeijer AN, Leung D, Huisman MC, Bahce I, Hoekstra OS, van Dongen GAMS, Boellaard R, du S, Hayes W, Smith R, Windhorst AD, Hendrikse NH, Poot A, Vugts DJ, Thunnissen E, Morin P, Lipovsek D, Donnelly DJ, Bonacorsi SJ, Velasquez LM, de Gruijl TD, Smit EF, de Langen AJ (2018) Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat Commun 9:4664
Huisman M, Niemeijer AL, Windhorst B, Schuit R, Leung D, Hayes W, Poot A, Bahce I, Radonic T, Oprea-Lager D, Hoekstra O, Thunnissen E, Hendrikse H, Smit E, de Langen J, Boellaard R (2020) Quantification of PD-L1 expression with [(18)F]BMS-986192 PET/CT in patients with advanced stage non-small-cell lung cancer. J Nucl Med:jnumed.119.240895
Knowles SM, Wu AM (2012) Advances in immuno-positron emission tomography: antibodies for molecular imaging in oncology. J Clin Oncol 30:3884–3892
Truillet C, Oh HLJ, Yeo SP, Lee CY, Huynh LT, Wei J, Parker MFL, Blakely C, Sevillano N, Wang YH, Shen YS, Olivas V, Jami KM, Moroz A, Jego B, Jaumain E, Fong L, Craik CS, Chang AJ, Bivona TG, Wang CI, Evans MJ (2018) Imaging PD-L1 expression with ImmunoPET. Bioconjug Chem 29:96–103
Moroz A, Lee CY, Wang YH, Hsiao JC, Sevillano N, Truillet C, Craik CS, Fong L, Wang CI, Evans MJ (2018) A preclinical assessment of (89)Zr-atezolizumab identifies a requirement for carrier added formulations not observed with (89)Zr-C4. Bioconjug Chem 29:3476–3482
Xu Y, Poggio M, Jin HY, Shi Z, Forester CM, Wang Y, Stumpf CR, Xue L, Devericks E, So L, Nguyen HG, Griselin A, Gordan JD, Umetsu SE, Reich SH, Worland ST, Asthana S, Barna M, Webster KR, Cunningham JT, Ruggero D (2019) Translation control of the immune checkpoint in cancer and its therapeutic targeting. Nat Med 25:301–311
Hu S, Shively L, Raubitschek A et al (1996) Minibody: a novel engineered anti-carcinoembryonic antigen antibody fragment (single-chain Fv-CH3) which exhibits rapid, high-level targeting of xenografts. Cancer Res 56:3055–3061
De Silva RA, Kumar D, Lisok A et al (2018) Peptide-based (68)Ga-PET radiotracer for imaging PD-L1 expression in cancer. Mol Pharm 15:3946–3952
Hu K, Kuan H, Hanyu M, Masayuki H, Xie L, Zhang Y, Nagatsu K, Kotaro N, Suzuki H, Hisashi S, Zhang MR (2019) Developing native peptide-based radiotracers for PD-L1 PET imaging and improving imaging contrast by pegylation. Chem Commun (Camb) 55:4162–4165
Wissler HL, Ehlerding EB, Lyu Z, Zhao Y, Zhang S, Eshraghi A, Buuh ZY, McGuth JC, Guan Y, Engle JW, Bartlett SJ, Voelz VA, Cai W, Wang RE (2019) Site-specific immuno-PET tracer to image PD-L1. Mol Pharm 16:2028–2036
Donnelly DJ, Smith RA, Morin P, Lipovšek D, Gokemeijer J, Cohen D, Lafont V, Tran T, Cole EL, Wright M, Kim J, Pena A, Kukral D, Dischino DD, Chow P, Gan J, Adelakun O, Wang XT, Cao K, Leung D, Bonacorsi SJ Jr, Hayes W (2018) Synthesis and biologic evaluation of a novel (18)F-labeled adnectin as a PET radioligand for imaging PD-L1 expression. J Nucl Med 59:529–535
Mayer AT, Natarajan A, Gordon SR, Maute RL, McCracken MN, Ring AM, Weissman IL, Gambhir SS (2017) Practical immuno-PET radiotracer design considerations for human immune checkpoint imaging. J Nucl Med 58:538–546
Funding
M.J.E. was supported by an American Cancer Society Research Scholar Grant (130635-RSG-17-005-01-CCE) and the National Institute Biomedical Imaging and Bioengineering (R01EB025207). L.F. was supported by a Prostate Cancer Foundation Challenge Grant and the National Cancer Institute (R01CA223484, U01CA233100, and U01CA244452). C.S.C. was supported by the National Cancer Institute (P41CA196276). D.R. was supported by the National Cancer Institute (R35CA242986). Small animal PET/CT studies were performed on the instrument supported by National Institutes of Health grant S10RR023051. Research from UCSF reported in this publication was supported in part by the National Cancer Institute of the National Institutes of Health under Award Number P30CA082103. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Research from Singapore Immunology Network in this publication was supported by the Category 3 Industrial Alignment Fund (IAF 311007) awarded by the Biomedical Research Council of A*STAR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Ethical Approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 3269 kb)
Rights and permissions
About this article
Cite this article
Wei, J., Wang, Yh., Lee, C.Y. et al. An Analysis of Isoclonal Antibody Formats Suggests a Role for Measuring PD-L1 with Low Molecular Weight PET Radiotracers. Mol Imaging Biol 22, 1553–1561 (2020). https://doi.org/10.1007/s11307-020-01527-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-020-01527-3