Abstract
Background
Frameless neuronavigation allows neurosurgeons to visualize and relate the position of surgical instruments to intracranial pathologies based on preoperative tomographic imaging. However, neuronavigation can often be inaccurate. Multiple factors have been proposed as potential causes, and new technologies are needed to overcome these challenges.
Objective
To evaluate the accuracy of neuronavigation systems compared to near-infrared (NIR) fluorescence imaging using Second Window Indocyanine Green, a novel technique, and to determine factors that lead to neuronavigation errors.
Methods
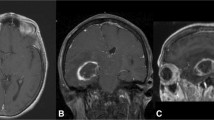
A retrospective analysis was conducted on 56 patients who underwent primary resections of intracranial tumors. Patients received 5 mg/kg ICG approximately 24 h preoperatively. Intraoperatively, neuronavigation was used to plan craniotomies to place the tumors in the center. After craniotomy, NIR imaging visualized tumor-specific NIR signals. The accuracy of neuronavigation and NIR fluorescence imaging for delineating the tumor boundary prior to durotomy was compared.
Results
The neuronavigation centers and NIR centers were 23.0 ± 7.7 % and 2.6 ± 1.1 % deviated from the tumor centers, respectively, relative to the craniotomy sizes. In 12 cases, significant changes were made to the planned durotomy based on NIR imaging. Patient position was a significant predictor of neuronavigation inaccuracy on both univariate and multivariate analysis, with the prone position having significantly higher inaccuracy (29.2 ± 8.1 %) compared to the supine (16.2 ± 8.1 %, p value < 0.001) or the lateral (17.9 ± 5.1 %, p value = 0.003) positions.
Conclusion
Patient position significantly affects neuronavigation accuracy. Intraoperative NIR fluorescence imaging before durotomy offers an opportunity to readjust the neuronavigation image space to better align with the patient space.



Similar content being viewed by others
References
Bucci MK, Maity A, Ph D et al (2004) Near complete surgical resection predicts a favorable outcome in pediatric patients with nonbrainstem , malignant gliomas results from a single center in the magnetic resonance imaging era. Cancer. https://doi.org/10.1002/cncr.20422
Anderson TMD (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. Cancer 95:190–198
Tumor B, Francisco S (2011) An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115:3–8. https://doi.org/10.3171/2011.2.JNS10998
Thomas NWD, Sinclair J (2019) Image-guided neurosurgery : history and current clinical applications. J Med Imaging Radiat Sci 46(3):331–342. https://doi.org/10.1016/j.jmir.2015.06.003
Ferrant M, Nabavi A, Macq B et al (2002) Serial registration of intraoperative MR images of the brain. Med Image Anal 6:337–359
Resection BC (2017) Clinical Application of Multimodal Neuronavigation System in Neuroendoscope-Assisted Skull. J Craniofac Surg 28(6):554–557. https://doi.org/10.1097/SCS.0000000000003859
Murray W, Enberger HK (1986) A frameless stereotaxic integration of computerized tomographic imaging and the operating microscope. J Neurosurg 65:545–549
Enchev Y (2009) Neuronavigation: geneology, reality, and prospects. Neurosurg Focus 27(3):E11. https://doi.org/10.3171/2009.6.focus09109
Orringer DA, Golby A, Jolesz F (2012) Neuronavigation in the surgical management of brain tumors: current and future trends. Expert Rev Med Devices 9(5):491–500. https://doi.org/10.1586/erd.12.42
Rainer Wirtz WS, Albert FK, Schwaderer M et al (2016) The benefit of neuronavigation for neurosurgery analyzed by its impact on glioblastoma surgery. Neurol Res. https://doi.org/10.1080/01616412.2000.11740684
Willems PWA, Taphoorn MJB, Burger H, van der Sprenkel JWB, Tulleken CAF (2008) Effectiveness of neuronavigation in resecting solitary intracerebral contrast-enhancing tumors: a randomized controlled trial. J Neurosurg 104(3):360–368. https://doi.org/10.3171/jns.2006.104.3.360
Gerard IJ, Kersten-Oertel M, Petrecca K, Sirhan D, Hall JA, Collins DL (2017) Brain shift in neuronavigation of brain tumors: a review. Med Image Anal. https://doi.org/10.1016/j.media.2016.08.007
Wang MN, Song ZJ (2011) Classification and analysis of the errors in neuronavigation. Neurosurgery. https://doi.org/10.1227/NEU.0b013e318209cc45
Hartkens T, Hill DLG, Castellano-Smith AD et al (2003) Measurement and analysis of brain deformation during neurosurgery. IEEE Trans Med Imaging. https://doi.org/10.1109/TMI.2002.806596
Nimsky C, Ganslandt O, Kober H, Ph D, Buchfelder M, Fahlbusch R (2001) Intraoperative magnetic resonance imaging combined with neuronavigation : a new concept. Neurosurgery 48(5):1082–1089
Shah MN, Leonard JR, Inder G et al (2012) Intraoperative magnetic resonance imaging to reduce the rate of early reoperation for lesion resection in pediatric neurosurgery. J Neurosurg Pediatr. https://doi.org/10.3171/2011.12.peds11227
Senft C, Franz K, Ulrich CT et al (2010) Low field intraoperative MRI-guided surgery of gliomas: a single center experience. Clin Neurol Neurosurg. https://doi.org/10.1016/j.clineuro.2009.12.003
Bettag C (2019) Endoscopic fluorescence-guided resection increases radicality in glioblastoma surgery. Oper Neurosurg (Hagerstown):1–6. https://doi.org/10.1093/ons/opz082
Zhao S, Wu J, Wang C et al (2013) Intraoperative fluorescence-guided resection of high- grade malignant gliomas using 5-aminolevulinic acid – induced porphyrins : a systematic review and meta- analysis of prospective studies. PLoS One 8(5). https://doi.org/10.1371/journal.-pone.0063682
Stummer W, Pichlmeier U, Meinel T et al (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. https://doi.org/10.1016/S1470-2045(06)70665-9
Cherrick GR, Leevy CM, Charles S, Davidson CS (1960) Indocyanine green : observations on its physical properties , plasma decay, and hepatic extraction Find the latest version. J Clin Invest 39(4):592–600
Madajewski B, Judy BF, Mouchli A, Kapoor V, Holt D, Wang MD (2012) Intraoperative near-infrared imaging of surgical wounds after tumor resections can detect residual disease. Clin Cancer Res:5741–5752. https://doi.org/10.1158/1078-0432.CCR-12-1188
Lee JYK, Pierce JT, Zeh R, Cho SS, Salinas R, Nie S, Singhal S (2017) Intraoperative near-infrared optical contrast can localize brain metastases. World Neurosurg 106:120–130. https://doi.org/10.1016/j.wneu.2017.06.128
Cho SS, Salinas R, Lee JYK (2019) Indocyanine-green for fluorescence-guided surgery of brain tumors : evidence, techniques, and practical experience. Front Surg 6:1–13. https://doi.org/10.3389/fsurg.2019.00011
Cho SS, Zeh R, Pierce JT, Salinas R, Singhal S, Lee JYK (2018) Comparison of near-infrared imaging camera systems for intracranial tumor detection. Mol Imaging Biol 20:213–220. https://doi.org/10.1007/s11307-017-1107-5
Lee JYK, Thawani JP, Pierce J, Zeh R, Martinez-Lage M, Chanin M, Venegas O, Nims S, Learned K, Keating J, Singhal S (2016) Intraoperative near-infrared optical imaging can localize gadolinium-enhancing Gliomas during surgery. Neurosurgery. 79(6):856–871. https://doi.org/10.1227/NEU.0000000000001450
Lee JYK, Pierce JT, Thawani JP et al (2018) Near-infrared fluorescent image-guided surgery for intracranial meningioma. J Neurosurg 128:380–390. https://doi.org/10.3171/2016.10.JNS161636.380
Leksell L, Lindquist C, Adler JR, Leksell D, Jernberg B, Steiner L (1987) A new fixation device for the Leksell stereotaxic system. J Neurosurg 66(4):626–629. https://doi.org/10.3171/jns.1987.66.4.0626
Asano K, Katayama K, Kakuta K, Oyama K, Ohkuma H (2017) Assessment of the accuracy and errors of head-up display by an optical neuronavigation system in brain tumor surgery. Oper Neurosurg 13(1):23–35. https://doi.org/10.1093/ons/opw001
Li P, Qian R, Niu C, Fu X (2017) Impact of intraoperative MRI-guided resection on resection and survival in patient with gliomas: a meta-analysis. Curr Med Res Opin. https://doi.org/10.1080/03007995.2016.1275935
Kubben PL, ter Meulen KJ, Schijns OEMG, ter Laak-Poort M, van Overbeeke J, van Santbrink H (2011) Intraoperative MRI-guided resection of glioblastoma multiforme: a systematic review. Lancet Oncol 12(11):1062–1070. https://doi.org/10.1016/S1470-2045(11)70130-9
Kubben PL, Scholtes F, Schijns OEMG, ter Laak-Poort M, Teernstra OP, Kessels AG, van Overbeeke J, Martin DH, van Santbrink H (2014) Intraoperative magnetic resonance imaging versus standard neuronavigation for the neurosurgical treatment of glioblastoma: a randomized controlled trial. Surg Neurol Int 5(1):70. https://doi.org/10.4103/2152-7806.132572
Lee JYK, Cho SS, Zeh R et al (2018) Folate receptor overexpression can be visualized in real time during pituitary adenoma endoscopic transsphenoidal surgery with near-infrared imaging. J Neurosurg 129(2):390–403. https://doi.org/10.3171/2017.2.JNS163191
Cho SS, Zeh R, Pierce JT et al (2019) Folate receptor near-infrared optical imaging provides sensitive and specific intraoperative visualization of nonfunctional pituitary adenomas. Oper Neurosurg. 16(1):59–70. https://doi.org/10.1093/ons/opy034
Cho SS, Jeon J, Buch L, et al. (2018) Intraoperative near-infrared imaging with receptor-specific versus passive delivery of fluorescent agents in pituitary adenomas :1–11. doi:https://doi.org/10.3171/2018.7.JNS181642
Elliott JT, Marra K, Evans LT, Davis SC, Samkoe KS, Feldwisch J, Paulsen KD, Roberts DW, Pogue BW (2017) Simultaneous In Vivo fluorescent markers for perfusion, Protoporphyrin metabolism, and EGFR expression for optically guided identification of orthotopic glioma. Clin Cancer Res 23(9):2203–2212. https://doi.org/10.1158/1078-0432.CCR-16-1400
de Souza ALR, Marra K, Gunn J, Samkoe KS, Hoopes PJ, Feldwisch J, Paulsen KD, Pogue BW (2017) Fluorescent Affibody molecule administered in vivo at a microdose level labels EGFR expressing Glioma Tumor regions. Mol Imaging Biol 19(1):41–48. https://doi.org/10.1007/s11307-016-0980-7
Zanganeh S, Hamby CV, Backer MV, Backer JM Enhanced fluorescence diffuse optical tomography with indocyanine green- encapsulating liposomes targeted to receptors for vascular endothelial growth factor in tumor vasculature with indocyanine green-encapsulating liposomes. J Neurosurg. https://doi.org/10.1117/1.JBO.18.12.126014
Xu C, Kumavor PD, Zhu Q Indocyanine green enhanced co- registered diffuse optical tomography and photoacoustic tomography tomography and photoacoustic tomography. J Biomed Opt. https://doi.org/10.1117/1.JBO.18.12.126006
Sano K, Ohashi M, Kanazaki K et al (2017) Indocyanine Green-Labeled Polysarcosine for in vivo photoacoustic tumor imaging. Bioconjug Chem. https://doi.org/10.1021/acs.bioconjchem.6b00715
Funding
Supported in part by the Institute for Translational Medicine and Therapeutics of the Perelman School of Medicine at the University of Pennsylvania (JYKL). In addition, research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000003 (JKYL). In addition, research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number TL1TR001880 (SSC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Disclosures
None, except as stated above in Funding.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cho, S.S., Teng, C.W., Ramayya, A. et al. Surface-Registration Frameless Stereotactic Navigation Is Less Accurate During Prone Surgeries: Intraoperative Near-Infrared Visualization Using Second Window Indocyanine Green Offers an Adjunct. Mol Imaging Biol 22, 1572–1580 (2020). https://doi.org/10.1007/s11307-020-01495-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-020-01495-8