Abstract
Purpose
Whole-body (WB) dynamic positron emission tomography (PET) enables imaging of highly quantitative physiological uptake parameters beyond the standardized uptake value (SUV). We present a novel dynamic WB anthropomorphic PET simulation framework to assess the potential of 2-deoxy-2-[18F]fluoro-D-glucose ([18F]FDG) net uptake rate constant (Ki) imaging in characterizing tumor heterogeneity.
Procedures
Validated heterogeneous [18F]FDG tumor kinetics were modeled within the XCAT phantom (ground truth). Thereafter, static (SUV) and dynamic PET data were simulated and reconstructed, followed by indirect WB Patlak Ki imaging. Subsequently, we compared the methods of affinity propagation (AP) and automatic segmentation with active contour (MASAC) to evaluate the impact of tumor delineation. Finally, we extracted the metabolically active tumor volume (MATV), Dice similarity coefficient (DSC), and the intratumoral heterogeneity metrics of the area under the cumulative intensity histogram curve (CIHAUC), homogeneity, entropy, dissimilarity, high-intensity emphasis (HIE), and zone percentage (ZP), along with the target-to-background (TBR) and contrast-to-noise ratios (CNR).
Results
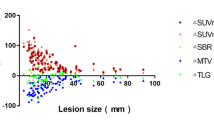
Ki images presented higher TBR but lower CNR compared to SUV. In contrast to MASAC, AP segmentation resulted in smaller bias for MATV and DSC scores in Ki compared to SUV images. All metrics, except for ZP, were significantly different in AP segmentation between SUV and Ki images, with significant correlation observed for MATV, homogeneity, dissimilarity, and entropy. With MASAC segmentation, CIHAUC, homogeneity, and dissimilarity were significantly different between SUV and Ki images, with all metrics, except for HIE and ZP, being significantly correlated. In ground truth images, increased heterogeneity was observed with Ki compared to SUV, with a high correlation for all metrics.
Conclusions
A novel simulation framework was developed for the assessment of the quantitative benefits of WB Patlak PET on realistic heterogeneous tumor models. Quantitative analysis showed that WB Ki imaging may provide enhanced TBR and facilitate lesion segmentation and quantification beyond the SUV capabilities.






Similar content being viewed by others
References
Chung MK, Jeong HS, Park SG, Jang JY, Son YI, Choi JY, Hyun SH, Park K, Ahn MJ, Ahn YC, Kim HJ, Ko YH, Baek CH (2009) Metabolic tumor volume of [18F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancer. Clin Cancer Res 15:5861–5868
La TH, Filion EJ, Turnbull BB et al (2009) Metabolic tumor volume predicts for recurrence and death in head-and-neck cancer. Int J Radiat Oncol Biol Phys 74:1335–1341
Obara P, Pu YL (2013) Prognostic value of metabolic tumor burden in lung cancer. Chin J Cancer Res 25:615–622
Kristian A, Revheim ME, Qu H, Mælandsmo GM, Engebråten O, Seierstad T, Malinen E (2013) Dynamic 18F-FDG-PET for monitoring treatment effect following anti-angiogenic therapy in triple-negative breast cancer xenografts. Acta Oncol 52:1566–1572
Dimitrakopoulou-Strauss A, Pan L, Strauss LG (2012) Quantitative approaches of dynamic FDG-PET and PET/CT studies (dPET/CT) for the evaluation of oncological patients. Cancer Imaging 12:283–289
Zaidi H, Karakatsanis N (2018) Towards enhanced PET quantification in clinical oncology. Br J Radiol 91:20170508
Karakatsanis NA, Casey ME, Lodge MA, Rahmim A, Zaidi H (2016) Whole-body direct 4D parametric PET imaging employing nested generalized Patlak expectation-maximization reconstruction. Phys Med Biol 61:5456–5485
Hatt M, Majdoub M, Vallieres M, Tixier F, le Rest CC, Groheux D, Hindie E, Martineau A, Pradier O, Hustinx R, Perdrisot R, Guillevin R, el Naqa I, Visvikis D (2015) 18F-FDG PET uptake characterization through texture analysis: investigating the complementary nature of heterogeneity and functional tumor volume in a multi-cancer site patient cohort. J Nucl Med 56:38–44
Tixier F, Vriens D, Cheze-Le Rest C et al (2016) Comparison of tumor uptake heterogeneity characterization between static and parametric 18F-FDG PET images in non-small cell lung cancer. J Nucl Med 57:1033–1039
Visser EP, Philippens ME, Kienhorst L et al (2008) Comparison of tumor volumes derived from glucose metabolic rate maps and SUV maps in dynamic 18F-FDG PET. J Nucl Med 49:892–898
Cheebsumon P, van Velden FH, Yaqub M et al (2011) Measurement of metabolic tumor volume: static versus dynamic FDG scans. EJNMMI Res 1:35
Segars WP, Sturgeon G, Mendonca S, Grimes J, Tsui BMW (2010) 4D XCAT phantom for multimodality imaging research. Med Phys 37:4902–4915
Vriens D, Disselhorst JA, Oyen WJG, de Geus-Oei LF, Visser EP (2012) Quantitative assessment of heterogeneity in tumor metabolism using FDG-PET. Int J Radiat Oncol Biol Phys 82:E725–E731
Dimitrakopoulou-Strauss A, Georgoulias V, Eisenhut M, Herth F, Koukouraki S, Mäcke HR, Haberkorn U, Strauss LG (2006) Quantitative assessment of SSTR2 expression in patients with non-small cell lung cancer using 68Ga-DOTATOC PET and comparison with 18F-FDG PET. Eur J Nucl Med Mol Imaging 33:823–830
Choi Y, Hawkins RA, Huang SC, Brunken RC, Hoh CK, Messa C, Nitzsche EU, Phelps ME, Schelbert HR (1994) Evaluation of the effect of glucose-ingestion and kinetic-model configurations of FDG in the normal liver. J Nucl Med 35:818–823
Lin KP, Huang SC, Choi Y, Brunken RC, Schelbert HR, Phelps ME (1995) Correction of spillover radioactivities for estimation of the blood time-activity curve from the imaged lv chamber in cardiac dynamic FDG PET studies. Phys Med Biol 40:629–642
Sachpekidis C, Mai EK, Goldschmidt H, Hillengass J, Hose D, Pan L, Haberkorn U, Dimitrakopoulou-Strauss A (2015) 18F-FDG dynamic PET/CT in patients with multiple myeloma patterns of tracer uptake and correlation with bone marrow plasma cell infiltration rate. Clin Nucl Med 40:E300–E307
Karakatsanis NA, Lodge MA, Tahari AK, Zhou Y, Wahl RL, Rahmim A (2013) Dynamic whole-body PET parametric imaging: I. Concept, acquisition protocol optimization and clinical application. Phys Med Biol 58:7391–7418
Feng D, Huang SC, Wang X (1993) Models for computer simulation studies of input functions for tracer kinetic modeling with positron emission tomography. Int J Biomed Comput 32:95–110
Le Maitre A, Segars WP, Marache S et al (2009) Incorporating patient-specific variability in the simulation of realistic whole-body F-18-FDG distributions for oncology applications. Proc IEEE 97:2026–2038
Wanet M, Lee JA, Weynand B, de Bast M, Poncelet A, Lacroix V, Coche E, Grégoire V, Geets X (2011) Gradient-based delineation of the primary GTV on FDG-PET in non-small cell lung cancer: a comparison with threshold-based approaches, CT and surgical specimens. Radiother Oncol 98:117–125
Daisne JF, Duprez T, Weynand B, Lonneux M, Hamoir M, Reychler H, Grégoire V (2004) Tumor volume in pharyngolaryngeal squamous cell carcinoma: comparison at CT, MR imaging, and FDG PET and validation with surgical specimen. Radiology 233:93–100
Loening AM, Gambhir SS (2003) AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging 2:131–137
Thielemans K, Tsoumpas C, Mustafovic S, Beisel T, Aguiar P, Dikaios N, Jacobson MW (2012) STIR: software for tomographic image reconstruction release 2. Phys Med Biol 57:867–883
Patlak CS, Blasberg RG, Fenstermacher JD (1983) Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab 3:1–7
Zaidi H, El Naqa I (2010) PET-guided delineation of radiation therapy treatment volumes: a survey of image segmentation techniques. Eur J Nucl Med Mol Imaging 37:2165–2187
Hatt M, Lee J, Schmidtlein CR et al (2017) Classification and evaluation strategies of auto-segmentation approaches for PET: report of AAPM Task Group No. 211. Med Phys 44:e1–e42
Zhuang M, Dierckx RA, Zaidi H (2016) Generic and robust method for automatic segmentation of PET images using an active contour model. Med Phys 43:4483–4494
Foster B, Bagci U, Ziyue X et al (2014) Segmentation of PET images for computer-aided functional quantification of tuberculosis in small animal models. IEEE Trans Biomed Eng 61:711–724
Zou KH, Warfield SK, Bharatha A, Tempany CMC, Kaus MR, Haker SJ, Wells WM III, Jolesz FA, Kikinis R (2004) Statistical validation of image segmentation quality based on a spatial overlap index—scientific reports. Acad Radiol 11:178–189
Hatt M, le Rest CC, Descourt P et al (2010) Accurate automatic delineation of heterogeneous functional volumes in positron emission tomography for oncology applications. Int J Radiat Oncol Biol Phys 77:301–308
van Griethuysen JJM, Fedorov A, Parmar C et al (2017) Computational radiomics system to decode the radiographic phenotype. Cancer Res 77:e104–e107
Frigge M, Hoaglin DC, Iglewicz B (1989) Some implementations of the boxplot. Am Stat 43:50–54
Karakatsanis NA, Lodge MA, Zhou Y, Wahl RL, Rahmim A (2013) Dynamic whole-body PET parametric imaging: II. Task-oriented statistical estimation. Phys Med Biol 58:7419–7445
Chen HW, Jiang JZ, Gao JL, Liu D, Axelsson J, Cui M, Gong NJ, Feng ST, Luo L, Huang B (2014) Tumor volumes measured from static and dynamic F-18-fluoro-2-deoxy-D-glucose positron emission tomography-computed tomography scan: comparison of different methods using magnetic resonance imaging as the criterion standard. J Comput Assist Tomogr 38:209–215
Ilan E, Sandstrom M, Velikyan I, Sundin A, Eriksson B, Lubberink M (2017) Parametric net influx rate images of 68Ga-DOTATOC and 68Ga-DOTATATE: quantitative accuracy and improved image contrast. J Nucl Med 58:744–749
Wangerin KA, Muzi M, Peterson LM, Linden HM, Novakova A, Mankoff DA, Kinahan PE (2017) A virtual clinical trial comparing static versus dynamic PET imaging in measuring response to breast cancer therapy. Phys Med Biol 62:3639–3655
Karakatsanis N, Lodge M, Fahrni G et al (2016) Simultaneous SUV/patlak-4D whole-body PET: a multi-parametric 4D imaging framework for routine clinical application. J Nucl Med 57(Suppl. 2):367
Karakatsanis N, Lodge M, Zhou Y et al (2015) Novel multi-parametric SUV/Patlak FDG-PET whole-body imaging framework for routine application to clinical oncology. J Nucl Med 56(Suppl. 3):625
Funding
This work was supported by the Swiss National Science Foundation under grant SNSF 320030_176052, the Swiss Cancer Research Foundation under Grant KFS-3855-02-2016, and an Open Grant (2014GDDSIPL-06) from the Key Laboratory of Digital Signal and Image Processing of Guangdong Province, Shantou University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 248 kb)
Rights and permissions
About this article
Cite this article
Zhuang, M., Karakatsanis, N.A., Dierckx, R.A.J.O. et al. Quantitative Analysis of Heterogeneous [18F]FDG Static (SUV) vs. Patlak (Ki) Whole-body PET Imaging Using Different Segmentation Methods: a Simulation Study. Mol Imaging Biol 21, 317–327 (2019). https://doi.org/10.1007/s11307-018-1241-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-018-1241-8