Abstract
Purpose
A shear stress-induced atherosclerosis mouse model was characterized for its expression of inflammation markers with focus on CD80. With this model, we evaluated two positron emission tomography (PET) radiotracers targeting CD80 as well as 2-deoxy-2-[18F]fluoro-d-mannose ([18F]FDM) in comparison with 2-deoxy-2-[18F]fluoro-d-glucose ([18F]FDG).
Procedure
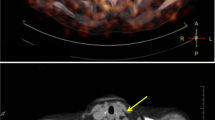
A flow constrictive cuff implanted around the common carotid artery in apolipoprotein E knockout mice resulted in plaque formation. CD80 expression levels and plaque histopathology were evaluated. Serial PET/X-ray computed tomography scans were performed to follow inflammation.
Results
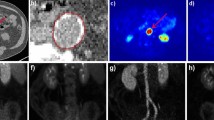
Plaque formation with increased levels of CD80 was observed. Histologically, plaques presented macrophage-rich and large necrotic areas covered by a thin fibrous cap. Of the CD80-specific tracers, one displayed an increased uptake in plaques by PET. Both [18F]FDG and [18F]FDM accumulated in atherosclerotic plaques.
Conclusion
This mouse model presented, similar to humans, an increased expression of CD80 which renders it suitable for non-invasively targeting CD80-positive immune cells and evaluating CD80-specific radiotracers.







Similar content being viewed by others
Abbreviations
- [18F]FDG:
-
2-deoxy-2-[18F]fluoro-D-glucose
- [18F]FDM:
-
2-deoxy-2-[18F]fluoro-D-mannose
- ApoE:
-
Apolipoprotein E
- DS:
-
Downstream
- FACS:
-
Fluorescence-activated cell sorting
- HFD:
-
High-fat diet
- KO:
-
Knockout
- ND:
-
Normal diet
- PET:
-
Positron emission tomography
- SUV:
-
Standardized uptake value
- US:
-
Upstream
References
Pedersen EM, Oyre S, Agerbaek M, et al. (1999) Distribution of early atherosclerotic lesions in the human abdominal aorta correlates with wall shear stresses measured in vivo. Eur J Vasc Endovasc Surg 18:328–333
Chiu JJ, Chien S (2011) Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev 91:327–387
Chatzizisis YS, Coskun AU, Jonas M, et al. (2007) Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol 49:2379–2393
Andreou I, Antoniadis AP, Shishido K, et al. (2015) How do we prevent the vulnerable atherosclerotic plaque from rupturing? Insights from in vivo assessments of plaque, vascular remodeling, and local endothelial shear stress. J Cardiovasc Pharmacol Ther 20:261–275
Rogers IS, Nasir K, Figueroa AL, et al. (2010) Feasibility of FDG imaging of the coronary arteries: comparison between acute coronary syndrome and stable angina. JACC Cardiovasc Imaging 3:388–397
Tawakol A, Migrino RQ, Bashian GG, et al. (2006) In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol 48:1818–1824
Gaemperli O, Shalhoub J, Owen DR, et al. (2012) Imaging intraplaque inflammation in carotid atherosclerosis with 11C-PK11195 positron emission tomography/computed tomography. Eur Heart J 33:1902–1910
Rominger A, Saam T, Vogl E, et al. (2010) In vivo imaging of macrophage activity in the coronary arteries using 68Ga-DOTATATE PET/CT: correlation with coronary calcium burden and risk factors. J Nucl Med 51:193–197
Beer AJ, Pelisek J, Heider P, et al. (2014) PET/CT imaging of integrin alphavbeta3 expression in human carotid atherosclerosis. JACC Cardiovasc Imaging 7:178–187
Dweck MR, Chow MW, Joshi NV, et al. (2012) Coronary arterial 18F-sodium fluoride uptake: a novel marker of plaque biology. J Am Coll Cardiol 59:1539–1548
Müller A, Mu L, Meletta R, et al. (2014) Towards non-invasive imaging of vulnerable atherosclerotic plaques by targeting co-stimulatory molecules. Int J Cardiol 174:503–515
Cheng C, Tempel D, van Haperen R, et al. (2006) Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 113:2744–2753
Cheng C, Tempel D, van Haperen R, et al. (2007) Shear stress-induced changes in atherosclerotic plaque composition are modulated by chemokines. J Clin Invest 117:616–626
Kuhlmann MT, Cuhlmann S, Hoppe I, et al. (2012) Implantation of a carotid cuff for triggering shear-stress induced atherosclerosis in mice. J Vis Exp.
Wenning C, Kloth C, Kuhlmann MT, et al. (2014) Serial F-18-FDG PET/CT distinguishes inflamed from stable plaque phenotypes in shear-stress induced murine atherosclerosis. Atherosclerosis 234:276–282
Winkel LC, Groen HC, van Thiel BS, et al. (2013) Folate receptor-targeted single-photon emission computed tomography/computed tomography to detect activated macrophages in atherosclerosis: can it distinguish vulnerable from stable atherosclerotic plaques? Mol Imaging 12:1–5
Tahara N, Mukherjee J, de Haas HJ, et al. (2014) 2-deoxy-2-[18F]fluoro-D-mannose positron emission tomography imaging in atherosclerosis. Nat Med 20:215–219
Maganto-Garcia E, Tarrio M, Lichtman AH (2012) Mouse models of atherosclerosis. Chapter 15: Unit 15 24 11–23.
Kapourchali FR, Surendiran G, Chen L, et al. (2014) Animal models of atherosclerosis. World J Clin Cases 2:126–132
Hartwig H, Silvestre-Roig C, Hendrikse J, et al. (2015) Atherosclerotic plaque destabilization in mice: a comparative study. PLoS One 10:e0141019
Lardenoye JH, Delsing DJ, de Vries MR, et al. (2000) Accelerated atherosclerosis by placement of a perivascular cuff and a cholesterol-rich diet in ApoE*3Leiden transgenic mice. Circ Res 87:248–253
Smedby O (1997) Do plaques grow upstream or downstream?: an angiographic study in the femoral artery. Arterioscler Thromb Vasc Biol 17:912–918
Ylä-Herttuala S, Bentzon JF, Daemen M, et al. (2011) Stabilisation of atherosclerotic plaques. Position paper of the European Society of Cardiology (ESC) Working Group on atherosclerosis and vascular biology. Thromb Haemost 106:1–19
van Bochove GS, Paulis LE, Segers D, et al. (2011) Contrast enhancement by differently sized paramagnetic MRI contrast agents in mice with two phenotypes of atherosclerotic plaque. Contrast Media Mol Imaging 6:35–45
van der Heiden K, Hoogendoorn A, Daemen MJ, Gijsen FJ (2016) Animal models for plaque rupture: a biomechanical assessment. Thromb Haemost 115:501–508
Winkel LC, Hoogendoorn A, Xing R, et al. (2015) Animal models of surgically manipulated flow velocities to study shear stress-induced atherosclerosis. Atherosclerosis 241:100–110
Bessell EM, Thomas P (1973) The deoxyfluoro-D-glucopyranose 6-phosphates and their effect on yeast glucose phosphate isomerase. Biochem J 131:77–82
Furumoto S, Shinbo R, Iwata R, et al. (2013) In vitro and in vivo characterization of 2-deoxy-2-18F-fluoro-D-mannose as a tumor-imaging agent for PET. J Nucl Med 54:1354–1361
Wolfs IM, Donners MM, de Winther MP (2011) Differentiation factors and cytokines in the atherosclerotic plaque micro-environment as a trigger for macrophage polarisation. Thromb Haemost 106:763–771
Chinetti-Gbaguidi G, Baron M, Bouhlel MA, et al. (2011) Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARgamma and LXRalpha pathways. Circ Res 108:985–995
Cho KY, Miyoshi H, Kuroda S, et al. (2013) The phenotype of infiltrating macrophages influences arteriosclerotic plaque vulnerability in the carotid artery. J Stroke Cerebrovasc Dis 22:910–918
Wilson HM (2010) Macrophages heterogeneity in atherosclerosis - implications for therapy. J Cell Mol Med 14:2055–2065
Acknowledgments
The authors thank Bruno Mancosu and Sabina Wunderlin for the technical support and helpful advice. We thank Dr. Michael Kuhlmann and Dirk Reinhardt from the European Institute for Molecular Imaging (EIMI, Münster, Germany) for introducing us to the shear stress-induced atherosclerosis mouse model and surgical training. We thank Dr. Cristina Müller (PSI, Villigen) for providing access to the Fuji ChemDri instrument and teaching sublingual blood collection. Ante Brekalo is acknowledged for the support in FDM synthesis. We appreciate the statistical advice by the statistical consultant of ETH. The authors acknowledge the support of the Scientific Center for Optical and Electron Microscopy (ScopeM) of the ETH Zurich. This work was financially supported by the Clinical Research Priority Program (CRPP) of the University of Zurich on Molecular Imaging (MINZ).
Author Contributions
R.M. conceived of and designed the experiments; established and performed all animal experiments, ex vivo staining, ex vivo PET, FACS, and qPCR; analyzed the data; and wrote the manuscript. L.S. performed the qPCR experiments and ex vivo staining and analyzed the data. N.B. supervised and analyzed the immunohistochemistry experiments. L.M. established the FDM chemistry and radiolabeling of 18F-FDM and was involved in the discussion of results. C.K. established and conducted all animal surgeries and in vivo PET scans. A.C. performed the organic synthesis of the AC74 precursor and reference compounds and radiolabeling of [18F]AC74. E.R. and C.H. conducted and analyzed the FACS experiments. S.M.A. and R.S. were involved in the discussion of results and revision of the manuscript. S.D.K. contributed to the supervision and planning of the experiments and revised the manuscript. A.M.H. supervised the study, experimental planning, and data interpretation and contributed to and supervised the manuscript writing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 2.24 mb)
Rights and permissions
About this article
Cite this article
Meletta, R., Steier, L., Borel, N. et al. CD80 Is Upregulated in a Mouse Model with Shear Stress-Induced Atherosclerosis and Allows for Evaluating CD80-Targeting PET Tracers. Mol Imaging Biol 19, 90–99 (2017). https://doi.org/10.1007/s11307-016-0987-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-016-0987-0