Abstract
Purpose
The purpose of this study was to quantify and model the longitudinal intra-tumor physiological response to a single dose of a monoclonal antibody specific to the VEGFR2 using dynamic contrast-enhanced CT.
Material and Methods
Dynamic contrast-enhanced CT imaging was performed on athymic nude mice bearing xenograft VEGF-transfected MCF-7 tumors (MCF7VEGF) to quantify intra-tumor physiology pre- and post-injection (days 2, 7, and 14) of a nonspecific (IgG1, controls) and specific (DC101, treated) monoclonal antibody targeting VEGFR2. Parametrical maps of tumor physiology—perfusion (F), permeability surface area (PS), fractional plasma (f p), and interstitial space (f is)—were obtained at four time points over a 2-week period.
Results
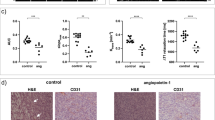
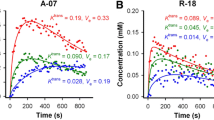
A temporal multistage recovery process whereby a decoupling of the fractional change in physiological parameters (f p, F) was observed when comparing treated to control tumors: f p and perfusion decreased by a combined 27% (P < 0.01) and 65% (P < 0.01) on day 2, while only perfusion remained reduced by 46% (P < 0.01) on day 7. Intra-tumor heterogeneity defined by the change in variance of perfusion decreased on days 2 and 7; no change in the variance of f p was observed. Analysis based on a mathematical model linking perfusion and vascular morphology indicates that a decrease in f p and perfusion was consistent with a reduction in blood vessel radius, followed by an increase in the vascular radius and tortuosity resulting in the decoupling of f p and perfusion before returning to control levels.
Conclusion
Inhibiting VEGFR2 activity results in a temporal decoupling of physiological parameters, which can be explained by a combination of morphological changes influencing perfusion. Such a decoupling has the potential to significantly impact the delivery of pharmaceuticals and oxygen within solid tumors, critical factors in combined anti-angiogenic and radio- and chemotherapies.








Similar content being viewed by others
References
Mayer RJ (2004) Two steps forward in the treatment of colorectal cancer. N Engl J Med 350(23):2406–2408
Hurwitz H, Fehrenbacher L, Novotny WF et al (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350(23):2335–2342
Kabbinavar FF, Schulz J, McCleod M et al (2005) Addition of bevacizumab to bolus 5-FU/leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol 23(16):3697–3705
Johnson DH, Fehrenbacher L, Novotny WF et al (2004) Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 22:2184–2191
Lyseng-Williamson KA, Robinson DM (2006) Spotlight on bevacizumab in advanced colorectal cancer, breast cancer, and non-small cell lung cancer. Biodrugs 20(3):193–195
Murata R, Nishimura Y, Hiraoka M (1997) An antiangiogenic agent (TNP-470) inhibited reoxygenation during fractionated radiotherapy of murine mammary carcinoma. Int J Radiat Oncol Biol Phys 37(5):1107–1113
Fenton BM, Paoni SF, Ding I (2004) Effect of VEGF receptor-2 antibody on vascular function and oxygenation in spontaneous and transplanted tumors. Radiother Oncol 72:221–230
Ma J, Li S, Reed K, Guo P, Gallo JM (2003) Pharmacodynamic-mediated effects of the angiogenesis inhibitor SU5416 on the tumor disposition of temozolomide in subcutaneous and intracerebral glioma xenograft models. J Pharmacol Exp Ther 305(3):833–839
Winkler F, Kozin SV, Tong RT et al (2004) Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metal loproteinases. Cancer Cell 6:553–563
Wildiers H, Guetens G, De Boeck G et al (2003) Effect of antivascular endothelial growth factor treatment on the intratumoral uptake of CPT-II. Br J Cancer 88(12):1979–1986
Pietras K, Stumm M, Hubert M et al (2003) STI571 enhances the therapeutic index of epothilone B by a tumor-selective increase of drug uptake. Clin Cancer Res 9(10):3779–3787
Yang AD, Bauer TW, Camp ER et al (2005) Improving delivery of antineoplastic agents with anti-vascular endothelial growth factor therapy. Cancer 103(8):1561–1570
Paez-Ribes M, Allen E, Hudock J et al (2009) Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 15:220–231
Ebos JML, Lee CR, Cruz-Munoz W et al (2009) Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 15:232–239
Bergers G, Hanahan D (2008) Models of resistance to anti-angiogenic therapy. Nat Rev 8:592–603
Grepin R, Pages G (2010) Molecular mechanisms of resistance to tumour anti-angiogenic strategies. J Oncol 2010:835680
You WK, McDonald DM (2008) The hepatocyte growth factor/c-Met signaling pathway as a therapeutic target to inhibit angiogenesis. BMB Reports 41(12):833–839
Nakagawa T, Tohyama O, Yamaguchi A et al (2010) E7050: a dual c-Met and VEGFR-2 tyrosine kinase inhibitor promotes tumor regression and prolongs survival in mouse xenograft models. Cancer Sci 101(1):210–215
Jain RK (2001) Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 7(9):987–989
Jain RK (2005) Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307:58–62
Jain RK (2003) Molecular regulation of vessel maturation. Nat Med 9:685–693
Kuszyk BS, Corl FM, Franano FN et al (2001) Tumor transport physiology: implications for imaging and imaging-guided therapy. Am J Roentgenol 177:747–753
Jain RK (1996) Whitaker Lecture: delivery of molecules, particles, and cells to solid tumors. Ann Biomed Eng 24:457–473
McGee MC, Mamner JB, Williams RF et al (2010) Improved intratumoral oxygenation through vascular normalization increases glioma sensitivity to ionizing radiation. Int J Radiat Biol Phys 76(5):1537–1545
Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK (2004) Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res 64:3731–3736
Li K-L, Wilmes LJ, Henry RG et al (2005) Heterogeneity in the angiogenic response of a BT474 human breast cancer to a novel vascular endothelial growth factor-receptor tyrosine kinase inhibitor: assessment by voxel analysis of dynamic contrast-enhanced MRI. J Magn Reson Imaging 22:511–519
Pollard RE, Broumas AR, Wisner ER, Vekich SV, Ferrara KW (2007) Quantitative contrast enhanced ultrasound and CT assessment of tumor response to antiangiogenic therapy in rats. Ultrasound Med Biol 33(2):235–245
Kan Z, Phongkitkarun S, Kobayashi S, Tang Y, Ellis LM, Lee TY (2005) Functional CT for quantifying tumor perfusion in antiangiogenic therapy in a rat model. Radiology 237:151–158
Gossmann A, Helbich TH, Kuriyama N et al (2002) Dynamic contrast-enhanced magnetic resonance imaging as a surrogate marker of tumor response to anti-angiogenic therapy in a xenograft model of glioblastoma multiforme. J Magn Reson Imaging 15:233–240
Checkley D, Tessier JJ, Kendrew J, Waterton JC, Wedge SR (2003) Use of dynamic contrast-enhanced MRI to evaluate acute treatment with ZD6474, a VEGF signaling inhibitor, in PC-3 prostate tumors. Br J Cancer 89:1889–1895
Morgan B, Thomas AL, Drevs J et al (2003) Dynamic contrast-enhanced magnetic resonance imaging as a biomarker for the pharmacological response of PTK787/ZK 222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases, in patients with advanced colorectal cancer and liver metastases: results from two phase I studies. J Clin Oncol 21:3955–3964
Liu G, Rugo HS, Wilding G et al (2005) Dynamic contrast-enhanced magnetic resonance imaging as a pharmacodynamic measure of response after acute dosing of AG-013736, an oral angiogenesis inhibitor, in patients with advanced solid tumors: results from a phase I study. J Clin Oncol 23:5464–5473
Gossmann A, Helbich TH, Mesiano S, Shames DM, Wendland MF, Roberts TP (2000) Magnetic resonance imaging in an experimental model of human ovarian cancer demonstrating altered microvascular permeability after inhibition of vascular endothelial growth factor. Am J Obstet Gynecol 183:956–963
Cao M, Liang Y, Miller KD, Stantz KM (2009) Developing DCE-CT to quantify intra-tumor heterogeneity in breast tumors with differing angiogenic phenotypes. IEEE Trans Med Imaging 28(6):861–871
Brix G, Bahner ML, Hoffmann U, Horvath A, Schreiber W (1999) Regional blood flow, capillary permeability, and compartmental volumes: measurement with dynamic CT—initial experience. Radiology 210:269–276
Miles KA (2003) Perfusion CT for the assessment of tumour vascularity: which protocol? Br J Radiol 76:S36–S42
Ng CS, Kodama Y, Mullani NA et al (2009) Tumor blood flow measured by perfusion computed tomography and 15O-labeled water positron emission tomography: a comparison study. J Comput Assist Tomogr 33(3):460–465
Ash L, Teknos TN, Gandhi D et al (2009) Head and neck squamous cell carcinoma: CT perfusion can help noninvasively predict intratumoral microvessel density. Radiology 251(2):422–428
Steen GR, Wilson DA, Bowser C et al (1989) 31P NMR spectroscopic and near infrared spectrophotometric studies of anesthetics on in vivo RIF-1 tumors. NMR Biomed 2(3):87–92
Jain RK (1988) Determinants of tumor blood flow: a review. Cancer Res 48:2641–2658
Fournier RL (2007) Basic transport phenomena in biomedical engineering, 2nd edn. Taylor & Francis, New York
Ramanujan S, Pluen A, McKee TD, Brown EB, Boucher Y, Jain RK (2002) Diffusion and convection in collagen gels: implications for transport in the tumor interstitium. Biophys J 83:1650–1660
Miller KD, Miller M, Mehrotra S et al (2006) A physiological imaging pilot study of breast cancer treated with AZD2171. Clin Cancer Res 12(1):281–288
Yuan F, Chen Y, Dellian M, Safabakhsh N, Ferrara N, Jain RK (1996) Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc Natl Acad Sci USA 93:14765–14770
Batchelor TT, Sorensen AG, di Tomasa E et al (2007) AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11:83–95
Dings RPM, Loren M, Heun H et al (2007) Scheduling of radiation with angiogenesis inhibitors anginex and avastin improves therapeutic outcome via vessel normalization. Clin Cancer Res 13(11):3395–3402
Vajkoczy P, Farhadi M, Gaumann A et al (2002) Microtumor growth initiates angiogenic sprouting with simultaneous expression of VEGF, VEGF receptor-2, and angiopoietin-2. J Clin Invest 109:777–785
Sevick EM, Jain RK (1989) Geometric resistance to blood flow in solid tumors perfused ex vivo: effects of tumor size and perfusion pressure. Cancer Res 49:3506–3512
Konerding MA, Steinberg F, van Ackern C, Budach V, Streffer C (1992) Vascular patterns of tumors: scanning and transmission electron microscopic studies on human xenografts. Strahlenther Onkol 168(9):444–452
Baish JW, Gazit Y, Berk DA, Nozue M, Baxter LT, Jain RK (1996) Role of tumor vascular architecture in nutrient and drug delivery: an invasion percolation-based network model. Mol Res 51(3):327–346
Tian S, Hayes AJ, Metheny-Barlow LJ, Li LY (2002) Stabilization of breast cancer xenograft tumour neovasculature by angiopoietin-1. Br J Cancer 86(4):645–651
Jun YD, Ahmad SA, Akagi Y et al (2000) Role of the tumor microenvironment in mediating response to anti-angiogenic therapy. Cancer Metastasis Rev 19(1–2):147–157
Boudreau N, Myers C (2003) Breast cancer-induced angiogenesis: multiple mechanisms and the role of the microenvironment. Breast Cancer Res 5(3):140–146
Monsky WL, Carreira CM, Tsuzuki Y, Gohongi T, Fukumura D, Jain RK (2002) Role of host microenvironment in angiogenesis and microvascular functions in human breast cancer xenografts: mammary fat pad versus cranial tumors. Clin Cancer Res 8:1008–1013
Vosseler S, Mirancea N, Bohlen P, Mueller MM, Fusenig NE (2005) Angiogenesis inhibition by vascular endothelial growth factor receptor-2 blockade reduces stromal matrix metalloproteinase expression, normalizes stromal tissue, and reverts epithelial tumor phenotype in surface heterotransplants. Cancer Res 65(4):1294–1305
Nakahara T, Norberg SM, Shalinsky DR, Hu-Lowe DD, McDonald DM (2006) Effect of inhibition of vascular endothelial growth factor signaling on distribution of extravasated antibodies in tumors. Cancer Res 66(3):1434–1445
Baluk P, Falcon BL, Hashizume H et al (2008) Tumor angiogenesis. Springer, Berlin, pp 557–576
Gaustad J-V, Simonsen TG, Brurberg KG et al (2009) Blood supply in melanoma xenografts is governed by the morphology of the supplying arteries. Neoplasia 11(3):277–285
Nakatsu MN, Sainson RCA, Perez-del-Pulgar S et al (2003) VEGF121 and VEGF165 regulate blood vessel diameter through vascular endothelial growth factor receptor 2 in an in vitro angiogenesis model. Lab Investig 83(12):1873–1885
Bussink J, Kaanders JH, Rijken PF, Raleigh JA, Van der Kogel AJ (2000) Changes in blood perfusion and hypoxia after irradiation of a human squamous cell carcinoma xenograft tumor line. Radiat Res 153(4):398–404
Stantz KMS, Cao N, Liu B, Cao M, Chin-Sinex H, Mendonca M, Li JJ (2010) Effects of radiation on tumor hemodynamics and NF-kappaB in breast tumors. Proc SPIE BiOS 7564:75641J-1-6
Sonveaux P, Dessy C, Brouet A et al (2002) Modulation of the tumor vasculature functionality by ionizing radiation accounts for tumor radiosensitization and promotes gene delivery. FASEB 16:1979–1981
Yu H, Su M-Y, Wang Z, Nalcioglu O (2002) A longitudinal study of radiation-induced changes in tumor vasculature by contrast-enhanced magnetic resonance imaging. Radiat Res 158:152–158
Ibuki Y, Goto R (2004) Ionizing radiation-induced macrophage activiation: augmentation of nitric oxide production and its significance. Cell Mol Biol 50:617–626
Moeller BU, Cao Y, Li CY, Dewhirst MW (2004) Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of re-oxygenation, free radicals, and stress granules. Cancer Cell 5:429–441
Madani I, DeNeve W, Mareel M (2008) Does ionizing radiation stimulate cancer invasion and metastasis? Bull Cancer 95(3):292–300
Kaliski A, Maggiorell L, Cengel KA et al (2002) Angiogenesis and tumor growth inhibition by a matrix metalloproteinase inhibitor targeting radiation-induced invasion. Mol Cancer Ther 4:1717–1728
Sonveaux P, Brouet A, Havaux X et al (2003) Irradiation-induced angiogenesis through the up-regulation of nitric oxide pathway: implication for tumor radiotherpay. Cancer Res 63:1012–1029
Vala I, Martins LR, Imaizumi N, Nunes RJ, Rino J et al (2010) Low doses of ionizing radiation promote tumor growth and metastasis by enhancing angiogenesis. PLoS ONE 5(6):e11222-1-13
Stantz KM, Liang Y, Hutchins G (2004) Kinematic modeling and its implication in longitudinal chemotherapy study of tumor physiology: ovarian xenograft mouse model and contrast-enhanced dynamic CT. Proc SPIE Medical Imaging 5369:769–779
Kruger RA, Kiser WL Jr, Reinecke DR et al (2003) Thermoacoustic optical molecular imaging of small animals. Mol Imaging 2(2):113–123
Stantz KM, Liu B, Cao M et al (2006) Evaluating dynamic contrast-enhanced and photoacoustic CT to assess intra-tumor heterogeneity in xenograft mouse models. Proc SPIE Medical Imaging 6143:489–500
Acknowledgments
This work was supported in part by the Indiana Genomics Initiative. The Indiana Genomics Initiative is supported in part by the Lilly Endowment.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Significance: DCE-CT provides a way to concurrently quantify four physiological parameters with high temporal and spatial resolution, and to do so longitudinally as a tumor progresses or in response to therapy. Few studies, if any, have systematically quantified the intra-tumor physiological response at various time points after a single dose of therapy has been applied, whether it is anti-angiogenic, radiation, or chemotherapy. To date, clinical doses are chosen to maximize therapeutic efficacy. These doses have been shown to significantly impact tumor biology and physiology, which can lead to adaptive responses associated with therapeutic resistance and metastasis. In this manuscript, DCE-CT was developed and used to quantify changes in tumor perfusion and vascular plasma volume over a couple of weeks after receiving a single cytostatic dose of DC101 relative to a nonspecific antibody. These set of experiments demonstrate an initial vascular response and a time-dependent recovery of vascular growth followed by perfusion. By studying the heterogeneous hemodynamic status of the tumor prior to and longitudinally after AAT, DCE-CT can be used to determine the optimal time and dose between anti-angiogenic and cytotoxic therapies and the time between successive combined anti-angiogenic plus cytotoxic therapies.
Rights and permissions
About this article
Cite this article
Stantz, K.M., Cao, M., Cao, N. et al. Monitoring the Longitudinal Intra-tumor Physiological Impulse Response to VEGFR2 Blockade in Breast Tumors Using DCE-CT. Mol Imaging Biol 13, 1183–1195 (2011). https://doi.org/10.1007/s11307-010-0441-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-010-0441-7