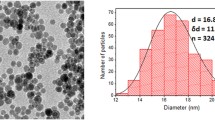
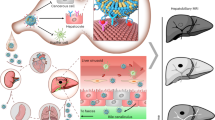
Abstract
Purpose
Magnetic resonance imaging (MRI) is a promising approach for non-invasive monitoring after liver cell transplantation. We compared in vitro labeling of human liver cells with nano-sized (SPIO) and micron-sized iron oxide particles (MPIO).
Procedures
The cellular iron load was quantified and phantom studies were performed using 3.0-T MRI. Transferrin receptor and ferritin gene expression, reactive oxygen species (ROS) formation, transaminase leakage, and urea synthesis were investigated over 6 days.
Results
Incubation with MPIO produced stronger signal extinctions in MRI at similar iron loads within shorter labeling time. MPIO had no negative effects on the cellular iron homeostasis or cell performance, whereas SPIO caused temporary ROS formation and non-physiologic activation of the iron metabolic pathway.
Conclusions
Our findings suggest that MPIO are suited for clinical translation of strategies for cellular imaging with MRI. Attention should be paid to iron release and oxidative stress caused by biodegradable contrast agents.




Similar content being viewed by others
References
Fitzpatrick E, Mitry RR, Dhawan A (2009) Human hepatocyte transplantation: state of the art. J Intern Med 266:339–357
Strom SC, Chowdhury JR, Fox IJ (1999) Hepatocyte transplantation for the treatment of human disease. Semin Liver Dis 19:39–48
Allen KJ, Mifsud NA, Williamson R, Bertolino P, Hardikar W (2008) Cell-mediated rejection results in allograft loss after liver cell transplantation. Liver Transplant 14:688–694
Dhawan A, Mitry RR, Hughes RD et al (2004) Hepatocyte transplantation for inherited factor VII deficiency. Transplantation 78:1812–1814
Bulte JW (2009) In vivo MRI cell tracking: clinical studies. AJR Am J Roentgenol 193:314–325
Modo M (2008) Noninvasive imaging of transplanted cells. Curr Opin Organ Transplant 13:654–658
Puppi J, Modo M (2009) Use of magnetic resonance imaging contrast agents to detect transplanted liver cells. Top Magn Reson Imaging 20:113–120
Weissleder R, Cheng HC, Bogdanova A, Bogdanov A Jr (1997) Magnetically labeled cells can be detected by MR imaging. J Magn Reson Imaging 7:258–263
Slotkin JR, Cahill KS, Tharin SA, Shapiro EM (2007) Cellular magnetic resonance imaging: nanometer and micrometer size particles for noninvasive cell localization. Neurotherapeutics 4:428–433
Modo M, Meade TJ, Mitry RR (2009) Liver cell labelling with MRI contrast agents. Methods Mol Biol 481:207–219
Morgul MH, Raschzok N, Schwartlander R et al (2008) Tracking of primary human hepatocytes with clinical MRI: initial results with Tat-peptide modified superparamagnetic iron oxide particles. Int J Artif Organs 31:252–257
Raschzok N, Morgul MH, Pinkernelle J et al (2008) Imaging of primary human hepatocytes performed with micron-sized iron oxide particles and clinical magnetic resonance tomography. J Cell Mol Med 12:1384–1394
Arbab AS, Wilson LB, Ashari P, Jordan EK, Lewis BK, Frank JA (2005) A model of lysosomal metabolism of dextran coated superparamagnetic iron oxide (SPIO) nanoparticles: implications for cellular magnetic resonance imaging. NMR Biomed 18:383–389
Hinds KA, Hill JM, Shapiro EM et al (2003) Highly efficient endosomal labeling of progenitor and stem cells with large magnetic particles allows magnetic resonance imaging of single cells. Blood 102:867–872
Crabbe A, Vandeputte C, Dresselaers T et al (2010) Effects of MRI contrast agents on the stem cell phenotype. Cell Transplant (in press)
Farrel E, Wielopolski P, Pavljasevic P et al (2008) Effects of iron oxide incorporation for long term cell tracking on MSC differentiation in vitro and in vivo. Biochem Biophys Res Commun 369:1076–1081
Janic B, Iskander AS, Rad AM, Soltanian-Zadeh H, Arbab AS (2008) Effects of ferumoxides-protamine sulfate labeling on immunomodulatory characteristics of macrophage-like THP-1 cells. PLoS ONE 3:e2499
Wang L, Deng J, Wang J et al (2009) Superparamagnetic iron oxide does not affect the viability and function of adipose-derived stem cells, and superparamagnetic iron oxide-enhanced magnetic resonance imaging identifies viable cells. Magn Reson Imaging 27:108–119
Nohroudi K, Arnhold S, Berhorn T, Addicks K, Hoehn M, Himmelreich U (2010) In vivo MRI stem cell tracking requires balancing of detection limit and cell viability. Cell Transplant 19(4):431–41
Schäfer R, Ayturan M, Bantleon R et al (2008) The use of clinically approved small particles of iron oxide (SPIO) for labeling of mesenchymal stem cells aggravates clinical symptoms in experimental autoimmune encephalomyelitis and influences their in vivo distribution. Cell Transplant 17:923–941
Schäfer R, Kehlbach R, Müller M et al (2009) Labeling of human mesenchymal stromal cells with superparamagnetic iron oxide leads to a decrease in migration capacity and colony formation ability. Cytotherapy 11:68–78
Stroh A, Boltze J, Sieland K et al (2009) Impact of magnetic labeling on human and mouse stem cells and their long-term magnetic resonance tracking in a rat model of Parkinson disease. Mol Imaging 8:166–178
Papanikolaou G, Pantopoulos K (2005) Iron metabolism and toxicity. Toxicol Appl Pharmacol 202:199–211
Pawelczyk E, Arbab AS, Pandit S, Hu E, Frank JA (2006) Expression of transferrin receptor and ferritin following ferumoxides-protamine sulfate labeling of cells: implications for cellular magnetic resonance imaging. NMR Biomed 19:581–592
Hubert N, Lescoat G, Sciot R et al (1993) Regulation of ferritin and transferrin receptor expression by iron in human hepatocyte cultures. J Hepatol 18:301–312
Raschzok N, Billecke N, Kammer NN et al (2009) Quantification of cell labeling with micron-sized iron oxide particles using continuum source atomic absorption spectrometry. Tissue Eng Part C Methods 15:681–686
Gu JM, Lim SO, Oh SJ, Yoon SM, Seong JK, Jung G (2008) HBx modulates iron regulatory protein 1-mediated iron metabolism via reactive oxygen species. Virus Res 133:167–177
Bos C, Delmas Y, Desmoulière A et al (2004) In vivo MR imaging of intravascularly injected magnetically labeled mesenchymal stem cells in rat kidney and liver. Radiology 233:781–789
Cai J, Zhang X, Wang X, Li C, Liu G (2008) In vivo MR imaging of magnetically labeled mesenchymal stem cells transplanted into rat liver through hepatic arterial injection. Contrast Media Mol Imaging 3:61–66
Choi D, Kim JH, Lim M et al (2008) Hepatocyte-like cells from human mesenchymal stem cells engrafted in regenerating rat liver tracked with in vivo magnetic resonance imaging. Tissue Eng Part C Methods 14:15–23
Ju S, Teng GJ, Lu H et al (2007) In vivo MR tracking of mesenchymal stem cells in rat liver after intrasplenic transplantation. Radiology 245:206–215
Luciani A, Parouchev A, Smirnov P, Clement O et al (2008) In vivo imaging of transplanted hepatocytes with a 1.5-T clinical MRI system–initial experience in mice. Eur Radiol 18:59–69
Shapiro EM, Sharer K, Skrtic S, Koretsky AP (2006) In vivo detection of single cells by MRI. Magn Reson Med 55:242–249
Arbab AS, Bashaw LA, Miller BR, Jordan EK, Bulte JW, Frank JA (2003) Intracytoplasmic tagging of cells with ferumoxides and transfection agent for cellular magnetic resonance imaging after cell transplantation: methods and techniques. Transplantation 76:1123–1130
Schäfer R, Kehlbach R, Wiskirchen J et al (2007) Transferrin receptor upregulation: in vitro labeling of rat mesenchymal stem cells with superparamagnetic iron oxide. Radiology 244:514–523
Williams JB, Ye Q, Hitchens TK, Kaufman CL, Ho C (2007) MRI detection of macrophages labeled using micrometer-sized iron oxide particles. J Magn Reson Imaging 25:1210–1218
Aisen P, Enns C, Wessling-Resnick M (2001) Chemistry and biology of eukaryotic iron metabolism. Int J Biochem Cell Biol 33:940–959
Tong X, Kawabata H, Koeffler HP (2002) Iron deficiency can upregulate expression of transferrin receptor at both the mRNA and protein level. Br J Haematol 116:458–464
Andriopoulos B, Hegedüsch S, Mangin J et al (2007) Sustained hydrogen peroxide induces iron uptake by transferrin receptor-1 independent of the iron regulatory protein/iron-responsive element network. J Biol Chem 282:20301–20308
Hussain SP, Raja K, Amstad PA et al (2000) Increased p53 mutation load in nontumorous human liver of Wilson disease and hemochromatosis: oxyradical overload diseases. Proc Natl Acad Sci USA 97:12770–12705
Acknowledgments
The authors are grateful to Kerstin Nehls and Nora Brunner for the technical assistance, Virginia Ding-Reinelt for the MRI operational assistance, and Dr. Ulrich Gauger for the statistical analysis.
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raschzok, N., Muecke, D.A., Adonopoulou, M.K. et al. In Vitro Evaluation of Magnetic Resonance Imaging Contrast Agents for Labeling Human Liver Cells: Implications for Clinical Translation. Mol Imaging Biol 13, 613–622 (2011). https://doi.org/10.1007/s11307-010-0405-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-010-0405-y