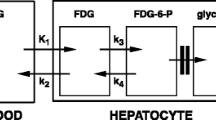
Abstract
Purpose
The intention here is to enhance the usefulness of the Gjedde–Patlak plot of dynamic positron emission tomography (PET) tracer uptake. Two additional parameters closely related to the physiologically significant and diagnostically useful phosphorylation rate k 3 are therefore studied. Additionally, their inter-institutional transportability is examined.
Methods
The two traditional parameters obtained from a Patlak plot are its slope Ki and its usually ignored tissue/plasma (=Q/Cp) axis intercept V. As a useful result, a normalized uptake rate may be defined as k = Ki /V. This is can be theoretically close to k 3. Similar to this an alternative normalized uptake rate is defined as k 3′ = Ki /V ′. Here, V ′ would be a composite of model rate constants, reasonably known a priori, and the measured V so as to depend less on errors in the latter. Parameter determination demonstrations utilize data from the 2-deoxy-2-[F-18]fluoro-D-glucose(FDG)-PET literature.
Results
Using median k i values from 24 FDG dynamic studies and algebraic relationships, on average: k = 1.07k 3 (r = 0.97), and k 3′ = 0.95k 3 (r = 0.91). A skeletal muscle case also demonstrates agreements with k 3. For liver malignancies k and k 3′ can be diagnostically slightly superior to Ki. Unaffected by institutionally dependent Q and Cp calibrations and methods, these can be more robust than Ki in a number of circumstances.
Conclusion
Two studied physiologically meaningful parameters, close to the diagnostically important k 3, can supplement Ki and enhance Patlak analysis by appropriately utilizing normally ignored information. Hitherto, k 3 was obtainable only by complex nonlinear least squares compartmental model analysis. The additional parameters can have more robust inter-institutional transportability than Ki.



Similar content being viewed by others
References
Hoeskstra CJ, Paglianiti I, Hoekstra OS et al. (2000) Monitoring response to therapy in cancer using [18F]-2-fluoro-2-deoxy-D-glucose and positron emission tomography: an overview of different analytical methods. Eur J Nucl Med 27:731-743
Graham MM, Peterson LM, Hayward RM. (2000) Comparison of simplified quantitative analyses of FDG uptake. Nucl Med Biol 27:647-655
Huang SC. (2000) Anatomy of SUV. Nucl Med Biol 20:643-646
Hunter GJ, Hamberg LM, Alpert NM, Choi NC, Fischman AJ. (1996) Simplified measurement of deoxyglucose utilization rate. J Nucl Med 37:950-955
Shiozaki T, Sadato N, Senda M et al. (2000) Noninvasive estimation of FDG input function for quantification of cerebral metabolic rate of glucose: optimization and multicenter evaluation. J Nucl Med 41:1612-1618
Wong W, Hicks K. (1994) A clinically practical method to acquire parametric images of unidirectional metabolic rates and blood spaces. J Nucl Med 35:1206-1212
Sundaram SK, Freedman NMT, Carrasquillo JA et al. (2004) Simplified kinetic analysis of tumor 18F-FDG uptake: a dynamic approach. J Nucl Med 45:1328-1333
Gjedde A. High- and low-affinity transport of D-glucose from blood to brain. (1981) J Neurochem 36:1463-1471
Patlak CS, Blasberg RG, Fenstermacher JD. (1983) Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab 3:1-7
Jones HA, Sriskandan S, Peters AM et al. (1997) Dissociation of neutrophil emigration and metabolic activity in lobar pneumonia and bronchiectasis. Eur Resp J 10:795-803
Chen DL, Mintun MA, Schuster DP. (2004) Comparison of methods to quantitate 18F-FDG uptake with PET during experimental acute lung injury. J Nucl Med 45:1583-1590
Juhasz C, Chugani DC, Muzik O et al. (2006) In vivo uptake and metabolism of α-[11C]methyl-L-tryptophan in human brain tumors. J Cereb Blood Flow Metab 26:345-357
Juhasz C, Muzik O, Lu Xin et al. (2009) Quantification of tryptophan transport and metabolism in lung tumors using PET. J Nucl Med 50:356-363
Thie JA. (2004) Understanding the standardized uptake value (SUV), its methods, and implications for usage. J Nucl Med 45:1431-1434
Boellaard R, Krak NC, Hoekstra OS, Lammertsma AA. (2004) Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: a simulation study. J Nucl Med 45:1519-1527
Park K, Ashlock R, Chang J et al. (2007) High variation in standardized uptake values among PET systems from different manufacturers. J Nucl Med 48:185P
Wang GJ, Volkow ND, Wolf AP et al. (1994) Intersubject variability of brain glucose metabolic measurements in young normal males. J Nucl Med 35:1457-1466
Gjedde A, Wienhard K, Heiss WD et al. (1985) Comparative regional analysis of 2-fluorodeoxyglucose and methylglucose uptake in brain of four stroke patients with special reference to the regional estimation of the lumped constant. J Cereb Blood Flow Metab 5:163-178
Bertoldo A, Peltoniemi P, Oikonen V et al. (2001) Kinetic modeling of [18F]FDG in skeletal muscle by PET: a four-compartment five-rate-constant model. Am J Physiol Endocrinol Metab 281:E524-E536
Okazumi S, Isono K, Enomoto K et al. (1992) Evaluation of liver tumors using Fluorine-18-fluorodeoxyglucose PET: characterization of tumor and assessment of effect of treatment. J Nucl Med 33:333-339
Jagust WJ, Seab JP, Huesman RH et al. (1991) Diminished glucose transport in Alzheimer’s disease: dynamic PET studies. J Cereb Blood Flow Metab 11:323-330
Minn H, Zasadny KR, Quint LE, Wahl RL. (1995) Lung cancer: reproducibility of quantitative measurements for evaluating 2-[F-18]-fluoro-2-deoxy-D-glucose uptake at PET. Radiology. 196:167-173
Torizuka T, Tamaki N, Inokuma T et al. (1995) In vivo assessment of glucose metabolism in hepatocellular carcinoma with FDG-PET. J Nucl Med 36:1811-1817
Wu HM, Huang SC, Choi Y, Hoh CK, Hawkins RA. (1995) A modeling method to improve quantitation of fluorodeoxyglucose uptake in heterogeneous tumor tissue. J Nucl Med. 36(2):297-306
Torizuka T, Zasadny KR, Recker B, Wahl RL. (1998) Untreated primary lung and breast cancers: correlation between F-18 FDG kinetic rate constants and findings of in vitro studies. Radiology 207:767-774
Reinhardt M, Beu M, Vosberg H et al. (1999) Quantification of glucose transport and phosphorylation in human skeletal muscle using FDG PET. J Nucl Med 40:977-985
Dimitrakopoulou-Strauss A, Strauss LG, Schwarzbach M et al. (2001) Dynamic PET 18F-FDG studies in patients with primary and recurrent soft-tissue sarcomas: impact on diagnosis and correlation with grading. J Nucl Med 42(5):713-720
Graham MM, Muzi M, Spence AM et al. (2002) The FDG lumped constant in normal human brain. J Nucl Med 43:1157-1166
Dimitrakopoulou-Strauss A, Strauss LG, Heichel T et al. (2002) The role of quantitative (18)F-FDG PET studies for the differentiation of malignant and benign bone lesions. J Nucl Med 43:510-518
Hattori N, Huang SC, Wu HM et al. (2004) Acute changes in regional cerebral (18)F-FDG kinetics in patients with traumatic brain injury. J Nucl Med 45(5):775-783
Tseng J, Dunnwald LK, Schubert EK et al. (2004) 18F-FDG kinetics in locally advanced breast cancer: correlation with tumor blood flow and changes in response to neoadjuvant chemotherapy. J Nucl Med 45:1829-1837
Spence AM, Muzi M, Mankoff DA et al. (2004) 18F-FDG PET of gliomas at delayed intervals: improved distinction between tumor and normal gray matter. J Nucl Med 45:1653-1659
Murakami M, Imahori Y, Kimura S et al. (2005) Positron emission tomography elucidates transport system and tumor proliferation in meningiomas. Oncol Rep 14:853-859
Dimitrakopoulou-Strauss A, Georgoulias V, Eisenhut M et al. (2006) Quantitative assessment of SSTR2 expression in patients with non-small cell lung cancer using (68)Ga-DOTATOC PET and comparison with (18)F-F-FDG PET. Eur J Nucl Med 33(7):823-830
Wang Y, Chiu E, Rosenberg J, Gambhir SS. (2007) Standardized uptake value atlas: characterization of physiological 2-deoxy-2-[18F]fluoro-D-glucose uptake in normal tissues. Mol Imaging Biol 9:83-90
Blasberg RG, Patlak CS, Fenstermacher JD. (1983) Selection of experimental conditions for the accurate determination of blood-brain transfer constants from single-time experiments: a theoretical analysis. J Cereb Blood Flow Metab 3:215-225
Basu S, Habib Z, Houseni M et al. (2007) Novel quantitative techniques for assessing regional and global function and structure based on modern imaging modalities: implications for normal variation, aging and diseased states. Sem Nucl Med 37:223-239
Gambhir SS, Schwaiger M, Huang SC et al. (1989) Simple noninvasive quantification method for measuring myocardial glucose utilization in humans employing positron emission tomography and Fluorine-18 deoxyglucose. J Nucl Med 30:359-366
Lodge MA, Lucas JD, Marsden PK et al. (1999) A PET study of 18FDG uptake in soft tissue masses. Eur J Nucl Med 26:22-30
Patlak CS, Blasberg RG. (1985) Graphical evaluation of blood-to-brain transfer constant from multiple-time uptake data: generalizations. J Cereb Blood Flow Metab 5:584-590
Boellaard R, Oyen W, Hoekstra CJ et al. (2008) The Netherlands protocol for standardization and quantification of FDG whole body PET studies in multi-center trials. Eur J Nucl Med Mol Imaging 35:2320-2333
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thie, J.A. Volume-Normalized Uptake Rates with Robust Transportability from PET Dual-time and Patlak Analyses. Mol Imaging Biol 12, 479–487 (2010). https://doi.org/10.1007/s11307-009-0280-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-009-0280-6