Abstract
Introduction
Gentian spotted bleaching disease (GSBD), a novel disease of unknown etiology, affects Gentiana triflora plants that are cultivated as ornamental flowers in Japan. This disease leads to the production of necrotic leaf spots, a delay in flowering, and has thus become a serious problem for gentian production.
Objectives
The objective of this study was to identify the cause of GSBD in G. triflora by analyzing differences between healthy and GSBD-affected leaves.
Method
Selected metabolite concentrations in healthy and GSBD-affected leaves were quantified using capillary electrophoresis and liquid chromatography-mass spectrometry, and statistically significant differences in metabolite concentrations were assessed. GSBD-affected metabolic pathways were identified followed by examination of pathway-related gene expression and enzyme activities. Furthermore, the effects of root hypoxia on metabolite concentrations and gene expression were investigated.
Results
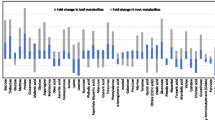
We found that concentrations of Calvin cycle intermediates and ribulose 1,5-bisphosphate carboxylase/oxygenase (RubisCO) activity were significantly lower in GSBD-affected leaves, whereas sucrose cleavage and Ala accumulation were enhanced. Since these metabolic changes are frequently observed in plants exposed to hypoxia, the expression of hypoxia-responsive genes was investigated. Expression levels of hypoxia-responsive genes were higher in GSBD-affected plants than in the controls. Furthermore, root hypoxia induced similar symptoms and metabolic changes as those observed in GSBD-affected plants.
Conclusion
Our results indicate that GSBD was likely induced by root hypoxia and that metabolome analysis is an effective tool for identifying the cause of plant disease with unknown etiologies.




Similar content being viewed by others
References
Abdel-Latif, A. (2008). Activity of sucrose synthase and acid invertase in wheat seedlings during a cold-shock using micro plate reader assays. Australian Journal of Basic and Applied Sciences, 2, 53–56.
Armengaud, P., Sulpice, R., Miller, A. J., Stitt, M., Amtmann, A., & Gibon, Y. (2009). Multilevel analysis of primary metabolism provides new insights into the role of potassium nutrition for glycolysis and nitrogen assimilation in Arabidopsis roots. Plant Physiology, 150, 772–785.
Ashraf, M., & Harris, P. J. C. (2013). Photosynthesis under stressful environments: An overview. Photosynthetica, 51, 163–190.
Atsumi, G., Tomita, R., Kobayashi, K., & Sekine, K. T. (2013). Establishment of an agroinoculation system for broad bean wilt virus 2. Archives of Virology, 158, 1549–1554.
Atsumi, G., Tomita, R., Yamashita, T., & Sekine, K. T. (2015). A novel virus transmitted through pollination causes ring-spot disease on gentian (Gentiana triflora) ovaries. The Journal of General Virology, 96, 431–439.
Bailey-Serres, J., & Voesenek, L. A. (2008). Flooding stress: acclimations and genetic diversity. Annual Review of Plant Biology, 59, 313–339.
Bieniawska, Z., Paul Barratt, D. H., Garlick, A. P., Thole, V., Kruger, N. J., Martin, C., et al. (2007). Analysis of the sucrose synthase gene family in Arabidopsis. The Plant Journal, 49, 810–828.
Cantu, M. D., Mariano, A. G., Palma, M. S., Carrilho, E., & Wulff, N. A. (2008). Proteomic analysis reveals suppression of bark chitinases and proteinase inhibitors in citrus plants affected by the citrus sudden death disease. Phytopathology, 98, 1084–1092.
Chaves, M. M., Flexas, J., & Pinheiro, C. (2009). Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Annals of Botany, 103, 551–560.
Chen, Y., Chen, X., Wang, H., Bao, Y., & Zhang, W. (2014). Examination of the leaf proteome during flooding stress and the induction of programmed cell death in maize. Proteome Science, 12, 33.
Ciereszko, I., & Kleczkowski, L. A. (2005). Expression of several genes in volved in sucrose/starch metabolism as affected by different strategies to induce phosphate deficiency in Arabidopsis. Acta Physiologiae Plantarum, 27, 147–155.
Cramer, G. R., Urano, K., Delrot, S., Pezzotti, M., & Shinozaki, K. (2011). Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biology, 11, 163.
Else, M. A., Janowiak, F., Atkinson, C. J., & Jackson, M. B. (2009). Root signals and stomatal closure in relation to photosynthesis, chlorophyll a fluorescence and adventitious rooting of flooded tomato plants. Annals of Botany, 103, 313–323.
Gimenez, C., Mitchell, V. J., & Lawlor, D. W. (1992). Regulation of photosynthetic rate of two sunflower hybrids under water stress. Plant Physiology, 98, 516–524.
Herbers, K., Meuwly, P., Frommer, W. B., Metraux, J. P., & Sonnewald, U. (1996). Systemic acquired resistance mediated by the ectopic expression of invertase: Possible hexose sensing in the secretory pathway. The Plant cell, 8, 793–803.
Hsu, F. C., Chou, M. Y., Peng, H. P., Chou, S. J., & Shih, M. C. (2011). Insights into hypoxic systemic responses based on analyses of transcriptional regulation in Arabidopsis. PLOS ONE, 6, e28888.
Imamura, T., Higuchi, A., Sekine, K. T., Yamashita, T., & Takahashi, H. (2015). High concentrations of sucrose induce overwintering bud formation in gentian plantlets cultured in vitro. Plant Biotechnology, 31, 97–104.
Kim, J. Y., Mahe, A., Brangeon, J., & Prioul, J. L. (2000). A maize vacuolar invertase, IVR2, is induced by water stress. Organ/tissue specificity and diurnal modulation of expression. Plant Physiology, 124, 71–84.
Kobayashi, K., Atsumi, G., Iwadate, Y., Tomita, R., Chiba, K., Akasaka, S., et al. (2013). Gentian Kobu-sho-associated virus: A tentative, novel double-strand RNA virus that is relevant to gentian Kobu-sho syndrome. Journal of General Plant Pathology, 79, 56–63.
Kobayashi, K., Atsumi, G., Yamaoka, N., & Sekine, K. T. (2012). Sequencing-based virus hunting and virus detection. Japan Agricultural Research Quarterly, 46, 123–128.
Kocal, N., Sonnewald, U., & Sonnewald, S. (2008). Cell wall-bound invertase limits sucrose export and is involved in symptom development and inhibition of photosynthesis during compatible interaction between tomato and Xanthomonas campestris pv vesicatoria. Plant Physiology, 148, 1523–1536.
Koch, K. (2004). Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Current Opinion in Plant Biology, 7, 235–246.
Krasensky, J., & Jonak, C. (2012). Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. Journal of Experimental Botany, 63, 1593–1608.
Kreuzwieser, J., Hauberg, J., Howell, K. A., Carroll, A., Rennenberg, H., Millar, A. H., et al. (2009). Differential response of gray poplar leaves and roots underpins stress adaptation during hypoxia. Plant Physiology, 149, 461–473.
Mehta, P., Jajoo, A., Mathur, S., & Bharti, S. (2010). Chlorophyll a fluorescence study revealing effects of high salt stress on Photosystem II in wheat leaves. Plant Physiology and Biochemistry, 48, 16–20.
Motohashi, R., & Myouga, F. (2015). Chlorophyll fluorescence measurements in Arabidopsis plants using a pulse-amplitude-modulated (PAM) fluorometer. Bio-protocol, 5, e1464. http://www.bio-protocol.org/e1464.
Nakatsuka, T., Yamada, E., Saito, M., Fujita, K., & Nishihara, M. (2013). Heterologous expression of gentian MYB1R transcription factors suppresses anthocyanin pigmentation in tobacco flowers. Plant Cell Reports, 32, 1925–1937.
Narsai, R., Rocha, M., Geigenberger, P., Whelan, J., & van Dongen, J. T. (2011). Comparative analysis between plant species of transcriptional and metabolic responses to hypoxia. The New phytologist, 190, 472–487.
Nouri, M. Z., Moumeni, A., & Komatsu, S. (2015). Abiotic stresses: insight into gene regulation and protein expression in photosynthetic pathways of plants. International Journal of Molecular Sciences, 16, 20392–20416.
Rocha, M., Licausi, F., Araujo, W. L., Nunes-Nesi, A., Sodek, L., Fernie, A. R., et al. (2010). Glycolysis and the tricarboxylic acid cycle are linked by alanine aminotransferase during hypoxia induced by waterlogging of Lotus japonicus. Plant Physiology, 152, 1501–1513.
Sturm, A., & Tang, G. Q. (1999). The sucrose-cleaving enzymes of plants are crucial for development, growth and carbon partitioning. Trends in Plant Science, 4, 401–407.
Takahashi, H., Fujita, K., Yoshida, C., & Nishihara, M. (2016). Metabolite profiling reveals the involvement of aberrant metabolic changes in Gentiana triflora seed showing poor germination. Journal of Horticultural Science & Biotechnology, 91, 148–155.
Takahashi, H., Imamura, T., Konno, N., Takeda, T., Fujita, K., Konishi, T., et al. (2014). The gentio-oligosaccharide gentiobiose functions in the modulation of bud dormancy in the herbaceous perennial Gentiana. The Plant cell, 26, 3949–3963.
Takahashi, H., Imamura, T., Miyagi, A., & Uchimiya, H. (2012). Comparative metabolomics of developmental alterations caused by mineral deficiency during in vitro culture of Gentiana triflora. Metabolomics, 8, 154–163.
Takahashi, H., Takahara, K., Hashida, S. N., Hirabayashi, T., Fujimori, T., Kawai-Yamada, M., et al. (2009). Pleiotropic modulation of carbon and nitrogen metabolism in Arabidopsis plants overexpressing the NAD kinase2 gene. Plant Physiology, 151, 100–113.
Takahashi, H., Watanabe, A., Tanaka, A., Hashida, S. N., Kawai-Yamada, M., Sonoike, K., et al. (2006). Chloroplast NAD kinase is essential for energy transduction through the xanthophyll cycle in photosynthesis. Plant and Cell Physiology, 47, 1678–1682.
Tauzin, A. S., & Giardina, T. (2014). Sucrose and invertases, a part of the plant defense response to the biotic stresses. Frontiers in Plant Science, 5, 293.
Wang, G., Kong, F., Zhang, S., Meng, X., Wang, Y., & Meng, Q. (2015). A tomato chloroplast-targeted DnaJ protein protects Rubisco activity under heat stress. Journal of experimental botany, 66, 3027–3040.
Waraich, E. A., Ahmad, R., Halim, A., & Aziz, T. (2012). Alleviation of temperature stress by nutrient management in crop plants: a review. Journal of Soil Science and Plant Nutrition, 12, 221–244.
Yanagisawa, H., Tomita, R., Katsu, K., Uehara, T., Atsumi, G., Tateda, C., et al. (2016). Combined DECS analysis and next-generation sequencing enable efficient detection of novel plant RNA viruses. Viruses, 8(3), 70
Yang, J. Y., Zheng, W., Tian, Y., Wu, Y., & Zhou, D. W. (2011). Effects of various mixed salt-alkaline stresses on growth, photosynthesis, and photosynthetic pigment concentrations of Medicago ruthenica seedlings. Photosynthetica, 49, 275–284.
Yordanova, R. Y., & Popova, L. P. (2007). Flooding-induced changes in photosynthesis and oxidative status in maize plants. Acta Physiologiae Plantarum, 29, 535–541.
Acknowledgements
This research was supported in part by a Grant-in-Aid for Scientific Research (B) (No. 15H04454) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, and by Iwate Prefecture. The authors wish to thank Dr. Masahiro Nishihara and Mr. Zenbi Naitoh for providing helpful advice and Ms. Chiharu Yoshida, Ms. Ayumi Obara, Ms. Yuko Kanno, and Ms. Sayaka Fujisaki for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Takahashi, H., Abe, H., Fujita, K. et al. The use of metabolome analysis to identify the cause of an unexplained disease of Japanese gentians (Gentiana triflora). Metabolomics 13, 51 (2017). https://doi.org/10.1007/s11306-017-1192-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-017-1192-0