Abstract
Background and aims
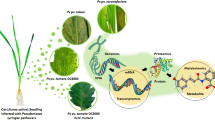
Water availability is well known for impacting productivity of Eucalyptus but comprehensive knowledge on cellular pathways involved in recovery and tolerance is scarce. In this context, we aimed to unveil putative mechanisms that account for drought recovery of E. globulus, and to identify specific strategies that make a clone more adapted to water deficit.
Methods
We resorted to comparative proteome (using difference gel electrophoresis) and metabolome [using Gas chromatography–mass spectrometry (GC–MS)] analyses in two E. globulus clones that exhibit physiological differences in their capacity to tolerate water shortage and restoration; also, interpretable networks were constructed coupled with previously assessed physiological matrices in order to interrogate the large datasets generated and develop a clear and integrative analysis.
Results
Our study enabled the separation and isolation of 2031 peptide spots, 217 of which were identified. GC–MS yielded the detection of 121 polar metabolites. Water shortage negatively affected photosynthesis, gene regulation, cell growth and secondary metabolites; enhanced photo protection, osmoprotection, and other defence-related pathways; and caused a shift from chloroplastic to mitochondrial energy generation. Recovery was characterised by upregulation of all previously described pathways. The analysis of the resilient clone AL-18, which presented a network very distinct from the responsive clone AL-10, reinforced the role of specific photosynthetic and defence-related proteins as key players in mediating drought tolerance and revealed new players: glutamine synthetase, malate dehydrogenase and isoflavone reductase-like protein.
Conclusion
This study provides a set of novel proteins and pathways involved in drought stress that represent potential drought tolerance markers for early selection of Eucalyptus.



Similar content being viewed by others
References
Asada, K. (2006). Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiology, 141(2), 391–396. doi:10.1104/pp.106.082040.
Bernard, S. M., & Habash, D. Z. (2009). The importance of cytosolic glutamine synthetase in nitrogen assimilation and recycling. New Phytologist, 182(3), 608–620.
Brossa, R., Pintó-Marijuan, M., Francisco, R., López-Carbonell, M., Chaves, M. M., & Alegre, L. (2015). Redox proteomics and physiological responses in Cistus albidus shrubs subjected to long-term summer drought followed by recovery. Planta, 241(4), 803–822. doi:10.1007/s00425-014-2221-0.
Chaves, M. M., Maroco, J. P., & Pereira, J. S. (2003). Understanding plant responses to drought-from genes to the whole plant. Functional Plant Biology, 30(3), 239–264. doi:10.1071/Fp02076.
Cheynier, V., Comte, G., Davies, K. M., Lattanzio, V., & Martens, S. (2013). Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiology and Biochemistry, 72, 1–20.
Coopman, R. E., Jara, J. C., Bravo, L. A., Sáez, K. L., Mella, G. R., & Escobar, R. (2008). Changes in morpho-physiological attributes of Eucalyptus globulus plants in response to different drought hardening treatments. Electronic Journal of Biotechnology, 11(2), 30–39.
Correia, B., Pintó-Marijuan, M., Castro, B. B., Brossa, R., López-Carbonell, M., & Pinto, G. (2014a). Hormonal dynamics during recovery from drought in two Eucalyptus globulus genotypes: From root to leaf. Plant Physiology and Biochemistry, 82, 151–160. doi:10.1016/j.plaphy.2014.05.016.
Correia, B., Pintó-Marijuan, M., Neves, L., Brossa, R., Dias, M. C., Costa, A., et al. (2014b). Water stress and recovery in the performance of two Eucalyptus globulus clones: Physiological and biochemical profiles. Physiologia Plantarum, 150(4), 580–592.
Costa e Silva, F., Shvaleva, A., Maroco, J. P., Almeida, M. H., Chaves, M. M., & Pereira, J. S. (2004). Responses to water stress in two Eucalyptus globulus clones differing in drought tolerance. Tree Physiology, 24(10), 1165–1172.
Feller, U., Anders, I., & Demirevska, K. (2008). Degradation of rubisco and other chloroplast proteins under abiotic stress. Gen Appl Plant Physiol, 34(1–2), 5–18.
Foyer, C. H., Rasool, B., Davey, J. W., & Hancock, R. D. (2016). Cross-tolerance to biotic and abiotic stresses in plants: A focus on resistance to aphid infestation. Journal of Experimental Botany, 67(7), 2025–2037.
Freeman, J. S., Potts, B. M., Downes, G. M., Pilbeam, D., Thavamanikumar, S., & Vaillancourt, R. E. (2013). Stability of quantitative trait loci for growth and wood properties across multiple pedigrees and environments in Eucalyptus globulus. New Phytologist, 198(4), 1121–1134. doi:10.1111/nph.12237.
Furuhashi, T., Fragner, L., Furuhashi, K., Valledor, L., Sun, X., & Weckwerth, W. (2012). Metabolite changes with induction of Cuscuta haustorium and translocation from host plants. Journal of Plant Interactions, 7(1), 84–93.
Galmés, J., Medrano, H., & Flexas, J. (2007). Photosynthetic limitations in response to water stress and recovery in mediterranean plants with different growth forms. New Phytologist, 175(1), 81–93.
González, I., Lé Cao, K.-A., & Déjean, S. (2011). mixOmics: Omics data integration Project. http://www.mixomics.org
Granda, V., Cuesta, C., Alvarez, R., Ordas, R., Centeno, M. L., Rodriguez, A., et al. (2011). Rapid responses of C14 clone of Eucalyptus globulus to root drought stress: Time-course of hormonal and physiological signaling. Journal of Plant Physiology, 168(7), 661–670. doi:10.1016/j.jplph.2010.09.015.
Kaminski, K. P., Kørup, K., Andersen, M. N., Sønderkær, M., Andersen, M. S., Kirk, H. G., et al. (2015). Cytosolic glutamine synthetase is important for photosynthetic efficiency and water use efficiency in potato as revealed by high-throughput sequencing QTL analysis. Theoretical and Applied Genetics, 128(11), 2143–2153.
Katam, R., Sakata, K., Suravajhala, P., Pechan, T., Kambiranda, D. M., Naik, K. S., et al. (2016). Comparative leaf proteomics of drought-tolerant and -susceptible peanut in response to water stress. Journal of Proteomics, 143, 209–226. doi:10.1016/j.jprot.2016.05.031.
Kottapalli, K. R., Rakwal, R., Shibato, J., Burow, G., Tissue, D., Burke, J., et al. (2009). Physiology and proteomics of the water-deficit stress response in three contrasting peanut genotypes. Plant, Cell and Environment, 32(4), 380–407.
Krasensky, J., & Jonak, C. (2012). Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. Journal of Experimental Botany, 63(4), 1593–1608.
Liu, D., Ford, K. L., Roessner, U., Natera, S., Cassin, A. M., Patterson, J. H., et al. (2013). Rice suspension cultured cells are evaluated as a model system to study salt responsive networks in plants using a combined proteomic and metabolomic profiling approach. Proteomics, 13(12–13), 2046–2062.
McKiernan, A. B., Hovenden, M. J., Brodribb, T. J., Potts, B. M., Davies, N. W., & O’Reilly-Wapstra, J. M. (2014). Effect of limited water availability on foliar plant secondary metabolites of two eucalyptus species. Environmental and Experimental Botany, 105, 55–64.
McKiernan, A. B., Potts, B. M., Brodribb, T. J., Hovenden, M. J., Davies, N. W., McAdam, S. A., et al. (2015). Responses to mild water deficit and rewatering differ among secondary metabolites but are similar among provenances within Eucalyptus species. Tree Physiology, 36(2), 133–147.
Pastore, D., Trono, D., Laus, M. N., Di Fonzo, N., & Flagella, Z. (2007). Possible plant mitochondria involvement in cell adaptation to drought stress a case study: Durum wheat mitochondria. Journal of Experimental Botany, 58(2), 195–210.
Printz, B., Sergeant, K., Lutts, S., Guignard, Cd, Renaut, J., & Hausman, J. F. (2013). From tolerance to acute metabolic deregulation: contribution of proteomics to dig into the molecular response of alder species under a polymetallic exposure. Journal of Proteome Research, 12(11), 5160–5179.
Sanchez, D. H., Siahpoosh, M. R., Roessner, U., Udvardi, M., & Kopka, J. (2008). Plant metabolomics reveals conserved and divergent metabolic responses to salinity. Physiologia Plantarum, 132(2), 209–219. doi:10.1111/j.1399-3054.2007.00993.x.
Scalabrin, E., Radaelli, M., Rizzato, G., Bogani, P., Buiatti, M., Gambaro, A., et al. (2015). Metabolomic analysis of wild and transgenic Nicotiana langsdorffii plants exposed to abiotic stresses: unraveling metabolic responses. Analytical and bioanalytical chemistry, 407(21), 6357–6368.
Sergeant, K., Spieß, N., Renaut, J., Wilhelm, E., & Hausman, J. F. (2011). One dry summer: A leaf proteome study on the response of oak to drought exposure. Journal of proteomics, 74(8), 1385–1395.
Shvaleva, A. L., Silva, F. C. E., Breia, E., Jouve, J., Hausman, J. F., Almeida, M. H., et al. (2006). Metabolic responses to water deficit in two Eucalyptus globulus clones with contrasting drought sensitivity. Tree Physiology, 26(2), 239–248. doi:10.1093/treephys/26.2.239.
Tomaz, T., Bagard, M., Pracharoenwattana, I., Lindén, P., Lee, C. P., Carroll, A. J., et al. (2010). Mitochondrial malate dehydrogenase lowers leaf respiration and alters photorespiration and plant growth in Arabidopsis. Plant Physiology, 154(3), 1143–1157.
Valdés, A. E., Irar, S., Majada, J. P., Rodriguez, A., Férnandez, B., & Pages, M. (2013). Drought tolerance acquisition in Eucalyptus globulus (Labill.): A research on plant morphology, physiology and proteomics. Journal of Proteomics, 79, 263–276. doi:10.1016/j.jprot.2012.12.019.
Valledor, L., Furuhashi, T., Hanak, A.-M., & Weckwerth, W. (2013). Systemic cold stress adaptation of Chlamydomonas reinhardtii. Molecular and Cellular Proteomics, 12(8), 2032–2047.
Valledor, L., & Jorrin, J. (2011). Back to the basics: Maximizing the information obtained by quantitative two dimensional gel electrophoresis analyses by an appropriate experimental design and statistical analyses. Journal of Proteomics, 74(1), 1–18. doi:10.1016/j.jprot.2010.07.007.
Vítámvás, P., Prášil, I. T., Kosova, K., Planchon, S., & Renaut, J. (2012). Analysis of proteome and frost tolerance in chromosome 5A and 5B reciprocal substitution lines between two winter wheats during long-term cold acclimation. Proteomics, 12(1), 68–85.
Wade, L. J., Ghareyazie, B., & Bennett, J. (2002). Proteomic analysis of rice leaves during drought stress and recovery. Proteomics, 2, 1131–1145.
Warren, C. R., Aranda, I., & Cano, F. J. (2011a). Metabolomics demonstrates divergent responses of two Eucalyptus species to water stress. Metabolomics, 8(2), 186–200. doi:10.1007/s11306-011-0299-y.
Warren, C. R., Aranda, I., & Cano, F. J. (2011b). Responses to water stress of gas exchange and metabolites in Eucalyptus and Acacia spp. Plant, Cell and Environment, 34(10), 1609–1629. doi:10.1111/j.1365-3040.2011.02357.x.
Weckwerth, W., Wenzel, K., & Fiehn, O. (2004). Process for the integrated extraction, identification and quantification of metabolites, proteins and RNA to reveal their co-regulation in biochemical networks. Proteomics, 4(1), 78–83.
Wolosiuk, R. A., & Buchanan, B. B. (1978). Regulation of chloroplast phosphoribulokinase by the ferredoxin/thioredoxin system. Archives of Biochemistry and Biophysics, 189(1), 97–101. doi:10.1016/0003-9861(78)90119-4.
Zhang, N., & Portis, A. R. (1999). Mechanism of light regulation of Rubisco: A specific role for the larger Rubisco activase isoform involving reductive activation by thioredoxin-f. Proceedings of the National Academy of Sciences United States of America, 96(16), 9438–9443.
Acknowledgments
This research was supported by Fundo Europeu de Desenvolvimento Regional (FEDER) through Programa Operacional Fatores de Competitividade (COMPETE), and by National Funds through the Portuguese Foundation for Science and Technology (FCT) within the Project PTDC/AGR-CFL/112996/2009. FCT/MEC, through national funds, and co-funding by the FEDER, within the PT2020 Partnership Agreement and Compete 2020 provide financial support to Centre for Environmental and Marine Studies (CESAM – UID/AMB/50017). FCT also supported the fellowships of Barbara Correia (SFRH/BD/86448/2012) and Glória Pinto (SFRH/BPD/101669/2014). The James Hutton Institute receives support from by the Rural and Environment Science and Analytical Services Division of the Scottish Government. We thank Altri florestal for providing the plant material, and Lucinda Neves and Marta Pintó-Marijuan for technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no competing interests exist.
Ethical approval
This article does not contain any studies with human or animal subjects.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Correia, B., Valledor, L., Hancock, R.D. et al. Integrated proteomics and metabolomics to unlock global and clonal responses of Eucalyptus globulus recovery from water deficit. Metabolomics 12, 141 (2016). https://doi.org/10.1007/s11306-016-1088-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-016-1088-4