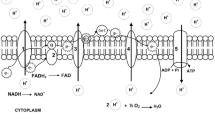
Abstract
This study investigated the physiological impact of changing electron donor–acceptor ratios on electron transfer pathways in the metabolically flexible subsurface bacterium Shewanella oneidensis, using batch and chemostat cultures, with an azo dye (ramazol black B) as the model electron acceptor. Altering the growth rate did result in changes in biomass yield, but not in other key physiological parameters including the total cytochrome content of the cells, the production of extracellular flavin redox shuttles or the potential of the organism to reduce the azo dye. Dramatic increases in the ability to reduce the dye were noted when cells were grown under conditions of electron acceptor (fumarate) limitation, although the yields of extracellular redox mediators (flavins) were similar under conditions of electron donor (lactate) or acceptor limitation. FT-IR spectroscopy confirmed shifts in the metabolic fingerprints of cells grown under these contrasting conditions, while spectrophotometric analyses supported a critical role for c-type cytochromes, expressed at maximal concentrations under conditions of electron acceptor limitation. Finally, key intracellular metabolites were quantified in batch experiments at various electron donor and acceptor ratios and analysed using discriminant analysis and a Bayesian network to construct a central metabolic pathway model for cells grown under conditions of electron donor or acceptor limitation. These results have identified key mechanisms involved in controlling electron transfer in Shewanella species, and have highlighted strategies to maximise reductive activity for a range of bioprocesses.




Similar content being viewed by others
References
Alm, E. J., Huang, K. H., Price, M. N., Koche, R. P., Keller, K., Dubchak, I. L., et al. (2005). The microbes online web site for comparative genomics. Genome Research, 15, 1015–1022.
Begley, P., Francis-McIntyre, S., Dunn, W. B., Broadhurst, D. I., Halsall, A., Tseng, A., et al. (2009). Development and performance of a gas chromatography time-of-flight mass spectrometry analysis for large-scale nontargeted metabolomic studies of human serum. Analytical Chemistry, 81, 7038–7046.
Beliaev, A. S., Klingeman, D. M., Klappenbach, J. A., Wu, L., Romine, M. F., Tiedje, J. M., et al. (2005). Global transcriptome analysis of Shewanella oneidensis MR-1 exposed to different terminal electron acceptors. Journal of Bacteriology, 187, 7138–7145.
Berry, E. A., & Trumpower, B. L. (1987). Simultaneous determination of hemes a, b, and c from pyridine hemochrome spectra. Analytical Biochemistry, 161, 1–15.
Brown, M., Dunn, W. B., Dobson, P., Patel, Y., Winder, C. L., Francis-McIntyre, S., et al. (2009). Mass spectrometry tools and metabolite-specific databases for molecular identification in metabolomics. Analyst, 134, 1322–1332.
Brown, M., Dunn, W. B., Ellis, D. I., Goodacre, R., Handl, J., Knowles, J. D., et al. (2005). A metabolome pipeline: from concept to data to knowledge. Metabolomics, 1, 39–51.
Clarke, T. A., Edwards, M. J., Gates, A. J., Hall, A., White, G. F., Bradley, J., et al. (2011). Structure of a bacterial cell surface decaheme electron conduit. Proceedings of the National Academy of Sciences, 108, 9384–9389.
Coates, J. D., & Achenbach, L. A. (2002). The Biogeochemistry of Aquifer Systems: Manual of Environmental Microbiology. Washington, DC: ASM Press.
Cooper, G. F., & Herskovits, E. (1992). A Bayesian method for the induction of probabilistic networks from data. Machine Learning, 9, 309–347.
Cornish-Bowden, A., & Luz Cardenas, M. (2000). From genome to cellular phenotype: A role for metabolic flux analysis. Nature Biotechnology, 18, 267–268.
Correa, E., & Goodacre, R. (2011). A genetic algorithm-Bayesian network approach for the analysis of metabolomics and spectroscopic data: Application to the rapid identification of Bacillus spores and classification of Bacillus species. BMC Bioinformatics, 12, 33.
Coursolle, D., & Gralnick, J. A. (2010). Modularity of the Mtr respiratory pathway of Shewanella oneidensis strain MR-1. Molecular Microbiology, 77, 995–1008.
Dunn, W. B., Broadhurst, D. I., Atherton, H. J., Goodacre, R., & Griffin, J. L. (2011a). Systems level studies of mammalian metabolomes: The roles of mass spectrometry and nuclear magnetic resonance spectroscopy. Chemical Society Reviews, 40, 387–426.
Dunn, W. B., Broadhurst, D., Begley, P., Zelena, E., Francis-McIntyre, S., Anderson, N., et al. (2011b). Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nature Protocols, 6, 1060–1083.
Goodacre, R., Broadhurst, D., Smilde, A., Kristal, B., Baker, J., Beger, R., et al. (2007). Proposed minimum reporting standards for data analysis in metabolomics. Metabolomics, 3, 231–241.
Goodacre, R., Vaidyanathan, S., Dunn, W. B., Harrigan, G. G., & Kell, D. B. (2004). Metabolomics by numbers: Acquiring and understanding global metabolite data. Trends in Biotechnology, 22, 245–252.
Gorby, Y. A., Yanina, S., McLean, J. S., Rosso, K. M., Moyles, D., Dohnalkova, A., et al. (2006). Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proceedings of the National Academy of Sciences of the United States of America, 103, 11358–11363.
Heidelberg, J., Paulsen, I. T., Nelson, K. E., Gaidos, E. J., Nelson, W. C., Read, T. D., et al. (2002). Genome sequence of the dissimilatory metal ion-reducing bacterium Shewanella oneidensis. Nature Biotechnology, 20, 1118–1123.
Hernandez, M. E., & Newman, D. K. (2001). Extracellular electron transfer. Cellular and Molecular Life Sciences, 58, 1562–1571.
Hong, Y. G., Chen, X. J., Guo, J., Xu, Z. C., Xu, M. Y., & Sun, G. P. (2007). Effects of electron donors and acceptors on anaerobic reduction of azo dyes by Shewanella decolorationis S12. Applied Microbiology and Biotechnology, 74, 230–238.
Johnson, R. A., & Wichern, D. W. (2007). Applied multivariate statistical analysis (6th ed.). Pearson: Prentice Hall. ISBN: 0-13-187715-1.
Kopka, J., Schauer, N., Krueger, S., Birkemeyer, C., Usadel, B. R., Bergmaller, E., et al. (2005). GMD@CSB.DB: The Golm Metabolome Database. Bioinformatics, 21, 1635–1638.
Kudlich, M., Keck, A., Klein, J., & Stolz, A. (1997). Localization of the Enzyme System Involved in Anaerobic Reduction of Azo Dyes by Sphingomonas sp. Strain BN6 and Effect of Artificial Redox Mediators on the Rate of Azo Dye Reduction. Applied and Environment Microbiology, 63, 3691–3694.
Lovley, D. R., Coates, J. D., Blunt-Harris, E. L., Philips, E. J. P., & Woodward, J. C. (1996). Humic substances as electron for microbial respiration. Nature, 382, 445–448.
MacKenzie, D. A., Defernez, M., Dunn, W. B., Brown, M., Fuller, L. J., de Herrera, S. R. M. S., et al. (2008). Relatedness of medically important strains of Saccharomyces cerevisiae as revealed by phylogenetics and metabolomics. Yeast, 25, 501–512.
Marsili, E., Baron, D. B., Shikhare, I. D., Coursolle, D., Gralnick, J. A., & Bond, D. R. (2008). Shewanella secretes flavins that mediate extracellular electron transfer. Proceedings of the National Academy of Sciences, 105, 3968–3973.
Martens, H., & Stark, E. (1991). Extended multiplicative signal correction and spectral interference subtraction-new preprocessing methods for near infrared spectroscopy. Journal of Pharmaceutical and Biomedical Analysis, 9, 625–635.
Myers, C. R., & Myers, J. M. (1992). Localization of cytochromes to the outer membrane of anaerobically grown Shewanella putrefaciens MR-1. Journal of Bacteriology, 174, 3429–3438.
Myers, C. R., & Myers, J. M. (1993). Ferric reductase is associated with the membranes of anaerobically grown Shewanella putrefaciens MR-1. FEMS Microbiology Letters, 108, 15–21.
Myers, C. R., & Myers, J. M. (1997a). Isolation and characterization of a transposon mutant of Shewanella putrefaciens MR-1 deficient in fumarate reductase. Letters in Applied Microbiology, 25, 162–168.
Myers, C. R., & Myers, J. M. (1997b). Cloning and sequence of cymA, a gene encoding a tetraheme cytochrome c required for reduction of iron(III), fumarate and nitrate by Shewanella putrefaciens MR-1. Journal of Bacteriology, 179, 1143–1152.
Myers, J. M., & Myers, C. R. (2000). Role of tetraheme cytochrome CymA in anaerobic electron transport in cells of Shewanella putrefaciens MR-1 with normal levels of menaquinone. Journal of Bacteriology, 182, 67–75.
Myers, C. R., & Myers, J. M. (2004). Shewanella oneidensis MR-1 restores menaquinone synthesis to a menaquinone-negative mutant. Applied and Environmental Microbiology, 70, 5415–5425.
Myers, C. R., & Nealson, K. H. (1990). Respiration-linked proton translocation coupled to anaerobic reduction of manganese(IV) and iron(III) in Shewanella putrefaciens MR-1. Journal of Bacteriology, 172, 6232–6238.
Newman, D. K., & Kolter, R. (2000). A role for excreted quinones in extracellular electron transfer. Nature, 405, 94–97.
O’Hagan, S., Dunn, W. B., Brown, M., Knowles, J. D., & Kell, D. B. (2004). Closed-loop, multiobjective optimization of analytical instrumentation:gas chromatography/time-of-flight mass spectrometry of the metabolomes of human serum and of yeast fermentations. Analytical Chemistry, 77, 290–303.
Pearce, C. I., Christie, R., Boothman, C., von Canstein, H., Guthrie, J. T., & Lloyd, J. R. (2006). Reactive azo dye reduction by Shewanella strain J18 143. Biotechnology and Bioengineering, 95, 692–703.
Pearl, J. (1988). Probabilistic reasoning in intelligent systems: Networks of plausible inference. San Mateo, CA: Morgan Kaufmann.
Pearl, J. (2000). Causality: Models, reasoning and inference. Cambridge, UK: Cambridge University Press.
Rau, J., Knackmuss, H.-J., & Stolz, A. (2002). Effects of different quinoid redox mediators on the anaerobic reduction of azo dyes by bacteria. Environmental Science and Technology, 36, 1497–1504.
Reguera, G., McCarthy, K. D., Mehta, T., Nicoll, J. S., Tuominen, M. T., & Lovley, D. R. (2005). Extracellular electron transfer via microbial nanowires. Nature, 435, 1098–1101.
Robinson, T., McMullan, G., Marchant, R., & Nigam, P. (2001). Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresource Technology, 77, 247–255.
Russ, R., Rau, J., & Stolz, A. (2000). The function of cytoplasmic flavin reductases in the reduction of azo dyes by bacteria. Applied and Environmental Microbiology, 66, 1429–1434.
Schuetz, R., Kuepfer, L., & Sauer, U. (2007). Systematic evaluation of objective functions for predicting intracellular fluxes in Escherichia coli. Molecular Systems Biology, 3, 119.
Serres, M. H., & Riley, M. (2006). Genomic analysis of carbon source metabolism of Shewanella oneidensis MR-1: Predictions versus experiments. Journal of Bacteriology, 188, 4601–4609.
Sumner, L., Amberg, A., Barrett, D., Beale, M., Beger, R., Daykin, C., et al. (2007). Proposed minimum reporting standards for chemical analysis. Metabolomics, 3, 211–221.
Tang, Y. J., Chakraborty, R., Martin, H. G., Chu, J., Hazen, T. C., & Keasling, J. D. (2007a). Flux analysis of central metabolic pathways in geobacter metallireducens during reduction of soluble Fe(III)-nitrilotriacetic acid. Applied and Environmental Microbiology, 73, 3859–3864.
Tang, Y. J., Hwang, J. S., Wemmer, D. E., & Keasling, J. D. (2007b). Shewanella oneidensis MR-1 fluxome under various oxygen conditions. Applied and Environmental Microbiology, 73, 718–729.
Tang, Y. J., Meadows, A. L., Kirby, J., & Keasling, J. D. (2007c). Anaerobic central metabolic pathways in Shewanella oneidensis MR-1 reinterpreted in the light of isotopic metabolite labeling. Journal of Bacteriology, 189, 894–901.
Taylor, P., Pealing, S. L., Reid, G. A., Chapman, S. K., & Walkinshaw, M. D. (1999). Structural and mechanistic mapping of a unique fumarate reductase. Nature Structural Biology, 6, 1108–1112.
von Canstein, H., Ogawa, J., Shimizu, S., & Lloyd, J. R. (2008). Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Applied and Environmental Microbiology, 74, 615–623.
Wang, H., Hollywood, K., Jarvis, R. M., Lloyd, J. R., & Goodacre, R. (2010a). Phenotypic characterization of Shewanella oneidensis MR-1 under aerobic and anaerobic growth conditions by using Fourier Transform Infrared Spectroscopy and High-Performance Liquid Chromatography analyses. Applied and Environmental Microbiology, 76, 6266–6276.
Wang, H., Law, N., Pearson, G., van Dongen, B. E., Jarvis, R. M., Goodacre, R., et al. (2010b). Impact of silver(I) on the metabolism of Shewanella oneidensis. Journal of Bacteriology, 192, 1143–1150.
Winder, C. L., Dunn, W. B., & Goodacre, R. (2011). TARDIS-based microbial metabolomics: Time and relative differences in systems. Trends in Microbiology, 19, 315–322.
Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., et al. (2008). Global metabolic profiling of Escherichia coli cultures: An evaluation of methods for quenching and extraction of intracellular metabolites. Analytical Chemistry, 80, 2939–2948.
Acknowledgments
This research was supported by BBSRC grant BBS/B/03718 and a Manchester-CSC PhD scholarship to H Wang. Harald von Canstein and Georgios D. Antoniou are acknowledged for initial discussions on Shewanella physiology. E. C. and R. G. would like to thank the Seventh Framework Programme (CommonSense project—SEC-2010.1.3-3 ref: 261809) for financial support. RG and WD thank BBSRC and EPSRC for financial support of the Manchester Centre for Integrative Systems Biology.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, H., Correa, E., Dunn, W.B. et al. Metabolomic analyses show that electron donor and acceptor ratios control anaerobic electron transfer pathways in Shewanella oneidensis . Metabolomics 9, 642–656 (2013). https://doi.org/10.1007/s11306-012-0488-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11306-012-0488-3