Abstract
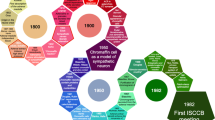
María Teresa Miras Portugal devoted most of her scientific life to the study of purinergic signalling. In an important part of her work, she used a model system: the chromaffin cells of the adrenal medulla. It was in these cells that she identified diadenosine polyphosphates, from which she proceeded to the study of adrenomedullary purinome: nucleotide synthesis and degradation, adenosine transport, nucleotide uptake into chromaffin granules, exocytotic release of nucleotides and autocrine regulation of chromaffin cell function via purinoceptors. This short review will focus on the current state of knowledge of the purinoceptors of adrenal chromaffin cells, a subject to which María Teresa made seminal contributions and which she continued to study until the end of her scientific life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In the beginning was dopamine beta-hydroxylase
María Teresa Miras Portugal began her scientific career by studying the properties of dopamine beta-hydroxylase (DBH) from adrenal chromaffin cells of the bovine adrenal medulla [1]. This is an enzyme located in both the soluble and membrane-bound fractions of chromaffin granules responsible for the final step of norepinephrine biosynthesis. The abundance of these granules (10,000–30,000 granules per cell) made the adrenal medulla a privileged tissue for the isolation and characterisation of the enzyme. In a series of articles with Dominique Aunis and Paul Mandel, carried out at the Institute of Neurochemistry in Strasbourg, where she did her doctoral thesis, she isolated the enzyme, characterised it structurally and kinetically [2, 3], and described its inhibition by 6-hydroxy-dopamine [4]. Back in Spain, María Teresa also undertook to characterise human DBH present in serum [5, 6] and to study its modifications in stress conditions such as hypoglycaemia and cold [7] or in streptozotocin-induced diabetes [8, 9]. The fact that this enzyme is released into the bloodstream together with catecholamines was one of the first biochemical proofs that this release takes place by the mechanism of exocytosis [10] and led at that time to investigate whether circulating levels of the enzyme were a good indicator of the activity of the adrenal medulla and peripheral adrenergic nerves [11,12,13].
From DBH to nucleotides
In addition to catecholamines and DBH, chromaffin granules also contain chromogranins and many different nucleotidic compounds. It was from the DBH of the chromaffin cells that María Teresa came into the purinergic field. Her initial interest was in purine metabolism, which led to the characterisation of adenine phosphoribosyltransferase, adenosine kinase [14, 15], and the study of adenosine transport including its positive modulation by ATP [16,17,18,19,20,21]. These studies coincided with the description of the presence of diadenosine polyphosphates (Ap4A, Ap5A) in the chromaffin granules, from where they are released together with the classical nucleotides (ATP, ADP) and the other components of the “secretory cocktail” [22, 23].
From nucleotides to purinoceptors
Traditionally, the high concentration of ATP in granules was thought to be due to its colligative properties, allowing the storage of large amounts of catecholamines with a limited increase in osmolarity [24]. However, ATP and the other purinergic nucleotides are not limited to this role; once released together with catecholamines, they contribute to the autocrine and paracrine regulation of the function of chromaffin cells and neighbouring endothelial cells through many different purinergic mechanisms and receptors. María Teresa was also a pioneer in the identification of these receptors, and although she gradually moved to the study of purinergic signalling in the central nervous system, both in physiological and pathological conditions, she never completely abandoned the adrenal medulla. She was not alone in this work, since one of her outstanding strengths was to surround herself with very capable collaborator—Magdalena Torres, Esmerilda G. Delicado, Enrique Castro, Jesús Pintor, Raquel Pérez-Sen—and the ability to establish scientific collaborations with other research groups. The first two articles in this line of research showed, on the one hand, the ability of cholinergic stimulation to induce the release of Ap4A and Ap5A from chromaffin cell cultures and perfused bovine adrenal glands, in a ratio vs ATP similar to that found in chromaffin granules [25] and, on the other hand, the effects of these compounds on the secretion of catecholamines by chromaffin cells [26]. In this respect, diadenosine polyphosphates (Ap3A, Ap4A and Ap5A) increase the basal release of catecholamines, this effect being strictly dependent on the presence of Ca2+ in the extracellular medium; they also inhibit the release of catecholamines induced by nicotinic stimulation and exert a dual stimulatory (Ap3A, Ap4A) or inhibitory (Ap5A) effect on the release induced by high extracellular K+. These two papers were quickly followed by another paper on Ap6A with similar findings [27].
The results published in these articles paved the way for the characterisation of the signalling mechanisms and purinergic receptors involved in the effects of diadenosine polyphosphates. Using Ca2+ microfluorometry with the fura-2 probe, it was shown that Ap4A and Ap5A increase the cytosolic Ca2+ concentration ([Ca2+]i) by promoting its release from intracellular pools. These effects would be mediated by a metabotropic P2Y receptor, since they were sensitive to depletion of internal Ca2+ stores by pretreatment with bradykinin or removal of extracellular Ca2+, mimicked and desensitised by the agonist adenosine 5′-O-(2-thiodiphosphate), and blocked by the P2Y receptor antagonist cibachrome blue [28]. The same P2Y receptor would also be responsible for the inhibition of Ca2+ influx induced by nicotinic stimulation (see mechanism of action below), leading to a reduction in catecholamine secretion, and the inhibition of adenosine transport by Ap4A, an effect that involves the activation of protein kinase C [28, 29].
A more detailed characterisation of purinergic receptors in bovine chromaffin cells was undertaken in collaboration with the group of Dr. Luis Rosário at the University of Coimbra. The results obtained by single cell microfluorometry of ([Ca2+]i showed a heterogeneous distribution of purinoceptors in bovine chromaffin cells, as well as a clear dependence on Ca2+ influx of ATP-induced ([Ca2+]i increase. The fact that ATP-sensitive cells also responded to 2-methylthioadenosine triphosphate (2MeSATP) and UTP with increases in [Ca2+]i was consistent with the expression of both P2X and P2Y receptors [30]. Likewise, bovine adrenal endothelial cells expressed a P2Y-type receptor coupled to Ca2+ mobilisation from intracellular pools. P2X receptor expression was confirmed in subsequent work by blocking ATP-induced [Ca2+]i elevations in UTP-insensitive cells with suramin; furthermore, in another subset of cells, ATP and UTP induced [Ca2+]i elevations that were insensitive to extracellular Ca2+ depletion and suramin, confirming P2Y receptor expression in bovine chromaffin cells. Notably, P2X receptor activation is coupled to preferential norepinephrine secretion, whereas P2Y receptor activation by itself does not induce a secretory response [31]; for a confirmation and extension of these studies, see [32, 33].
Coinciding with or immediately following the publication of this work, other research groups joined in the characterisation of purinergic receptors in chromaffin cells of several species (e.g. bovine, rat, guinea pig) [34]. In addition, the use of electrophysiological techniques allowed progress to be made in the study of P2X receptors, the modulation of voltage-gated Ca2+ channels by P2Y receptors and the effect of purinergic agonists on stimulated exocytosis through changes in cell capacitance.
In bovine chromaffin cells, ATP application reduces the Ca2+ current by inhibiting voltage-dependent Ca2+ channels (N, L, and P/Q-types). The inhibition is partially voltage-dependent (reversed by membrane depolarisation) and is mediated by a pertussis toxin (PTX)-sensitive G protein [35], suggesting the involvement of a P2Y receptor. This effect results in inhibition of exocytosis [36,37,38,39,40,41], providing a mechanism for the inhibition of catecholamine release previously described for purinergic agonists, including the diadenosine polyphosphates [26]. Of the various P2Y receptors expressed in chromaffin cells (P2Y1, P2Y2, P2Y4, P2Y6, P2Y12, P2Y13, and P2Y14) [42], the P2Y12 receptor mediates the effects on both voltage-gated Ca2+ channels and the secretory response [43]. The use of carbon fibre amperometry to record catecholamine release from individual vesicles showed that P2Y12 purinergic modulation reduces not only the probability of vesicle release but also the quantal content or amount of catecholamines released from each vesicle. This is a Giβγ protein-mediated effect that influences the opening time of the vesicle fusion pore, reducing it [44]. Notably, this effect is also voltage-dependent, such that membrane depolarisation reduces the effect on quantal size [42].
Few studies have investigated the expression of P2X receptors in chromaffin cells using electrophysiological techniques. For example, Otsuguro et al. [45] reported that ATP evoked an inward current in voltage-clamped guinea pig chromaffin cells with a reversal potential of approximately 0 mV, suggesting a non-selective cation permeability of the ATP-gated channel. As already mentioned for bovine chromaffin cells, ATP stimulation is coupled to catecholamine secretion and an increase in [Ca2+]i, both of which are suppressed by removal of extracellular Ca2+ [46]. These results were extended by Liu et al [47] who concluded that P2X receptors in guinea pig chromaffin cells share many characteristics of the P2X2 receptor subtype. Interestingly, rat chromaffin cells barely express P2X receptors under normal conditions. However, chromaffin cells from animals experiencing neuropathic pain, due to chronic sciatic nerve constriction, overexpress functional P2X3 and P2X7 receptors, which would be activated by released adenine nucleotides to increase cellular excitability (P2X3 receptor) or directly induce exocytosis (P2X7 receptor) [48, 49]. This unique example of purinoceptor plasticity in chromaffin cells was María Teresa’s last contribution to the purinergic field in this neuroendocrine cell, a cell model she used throughout her scientific career.
Concluding remarks
María Teresa soon came across the chromaffin cells of the adrenal medulla. Knowing their ability to release large quantities of catecholamines into the bloodstream, which, like Don Quixote, go on all sorts of adventures throughout the body, she soon realised that they had a faithful squire, adenine nucleotides, which, like Sancho, were responsible for ensuring good accommodation in the chromaffin granules and regulating their raids into the extracellular medium. María Teresa was able to extend many of her findings on chromaffin cells to the neurons of the central nervous system, both in physiological and pathological conditions (Alzheimer’s disease, Huntington’s disease, epilepsy, etc.), but she never completely abandoned the adrenal medulla. The authors of this article had the good fortune to accompany María Teresa on some of her scientific adventures. Let this account serve as a memorial and a tribute to her.
References
Aunis D, Miras-Portugal MT, Mandel P (1973) Bovine adrenal medullary dopamine beta-hydroxylase: purification by affinity chromatography, kinetic studies and presence of essential histidyl residues. Biochim Biophys Acta 1327(2):313–327. https://doi.org/10.1016/0005-2744(73)90414-2
Aunis D, Miras-Portugal MT, Mandel P (1974) Bovine adrenal medullary dopamine-beta-hydroxylase: studies on the structure. Biochim Biophys Acta 365(1):259–273. https://doi.org/10.1016/0005-2795(74)90270-0
Aunis D, Miras-Portugal MT, Mandel P (1975) Bovine adrenal medullary dopamine-beta-hydroxylase: studies on interaction with concanavalin A. J Neurochem 24(3):425–431. https://doi.org/10.1111/j.1471-4159.1975.tb07657.x
Aunis D, Miras-Portugal MT, Mandel P (1973) Inhibition of adrenal dopamine-beta-hydroxylase by 6-hydroxy dopamine. Biochem Pharmacol 22(20):2581–2589. https://doi.org/10.1016/0006-2952(73)90066-x
Miras-Portugal MT, Aunis D, Mandel P (1976) Human serum dopamine-beta-hydroxylase: purification, molecular weight, presence of sugars and kinetic properties. Biochimie 57(6-7):669–675. https://doi.org/10.1016/s0300-9084(75)80042-3
Miras-Portugal MT, Mandel P, Aunis D (1976) Amino acid and carbohydrate compositions of human serum dopamine-β-hydroxylase. Neurochem Res 1(4):403–408. https://doi.org/10.1007/BF00966231
García-Estañ J, Muñoz JA, Serrano MC, Carbonell LF, Miras Portugal MT, Quesada T (1985) Dopamine-beta-hydroxylase activity in adrenal gland and spleen of rats after fasting and cold exposure. Experientia 41(1):61–62
Muñoz JA, Garcia-Estañ J, Salom MG, Quesada T, Miras Portugal MT (1985) Sympathoadrenal activity and plasma glucose effects on plasma dopamine-beta-hydroxylase levels in rats. Clin Chim Acta 152(3):243–252. https://doi.org/10.1016/0009-8981(85)90099-3
Muñoz JA, García-Estañ J, Canteras M, Quesada T, Miras-Portugal MT (1986) Dopamine-beta-hydroxylase activity in plasma, spleen and adrenal gland of streptozotocin-diabetic rats: correlation with cataracts. Rev Esp Fisiol 42(1):63–70
Kirshner N, Kirshner AG (1981) Chromogranin A dopamine -hydroxylase and secretion from the adrenal medulla. Philos Trans R Soc Lond B Biol Sci 261(839):279–289. https://doi.org/10.1098/rstb.1971.0057
Geffen L (1984) Serum dopamine beta-hydroxylase as an index of sympathetic function. Life Sci 14(9):1593–1604. https://doi.org/10.1016/0024-3205(74)90262-8
Noth RH, Mulrow PJ (1976) Serum dopamine beta-hydroxylase as an index of sympathetic nervous system activity in man. Circ Res 38(1):1–5. https://doi.org/10.1161/01.res.38.1.1
Miras-Portugal MT, Galarza A, Díaz J, Santos-Ruiz A (1978) Estudios sobre la liberación de dopamina-beta-hidroxilasa por nicotina [Studies on the release of dopamine-beta-hydroxylase by nicotine (author's transl)]. Rev Esp Fisiol 34(1):9–13
Rotllán P, Miras-Portugal MT (1985) Purine nucleotide synthesis in adrenal chromaffin cells. J Neurochem 44(4):1029–1036. https://doi.org/10.1111/j.1471-4159.1985.tb08721.x
Rotllan P, Miras Portugal MT (1985) Adenosine kinase from bovine adrenal medulla. Eur J Biochem 151(2):365–371. https://doi.org/10.1111/j.1432-1033.1985.tb09110.x
Miras-Portugal MT, Torres M, Rotllan P (1986) Aunis D (1986) Adenosine transport in bovine chromaffin cells in culture. J Biol Chem. 261(4):1712–1719
Torres M, Molina P, Miras-Portugal MT (1986) Adenosine transporters in chromaffin cells. Quantification by dipyridamol monoacetate. FEBS Lett 201(1):124–128. https://doi.org/10.1016/0014-5793(86)80583-x
Torres M, Delicado EG, Miras-Portugal MT (1988) Adenosine transporters in chromaffin cells: subcellular distribution and characterization. Biochim Biophys Acta 969(2):111–120. https://doi.org/10.1016/0167-4889(88)90066-3
Torres M, Fideu MD, Miras-Portugal MT (1990) All nucleoside transporters in bovine chromaffin cells are nitrobenzylthioinosine sensitive. Neurosci Lett 112(2-3):343–347. https://doi.org/10.1016/0304-3940(90)90228-2
Casillas T, Delicado EG, Miras-Portugal MT (1993) Adenosine 5'-triphosphate modulation of nitrobenzylthioinosine binding sites in plasma membranes of bovine chromaffin cells. Neurosci Lett 164(1-2):51–54. https://doi.org/10.1016/0304-3940(93)90855-f
Delicado EG, Casillas T, Sen RP, Miras-Portugal MT (1984) Evidence that adenine nucleotides modulate nucleoside-transporter function. Characterization of uridine transport in chromaffin cells and plasma membrane vesicles. Eur J Biochem 225(1):355–362. https://doi.org/10.1111/j.1432-1033.1994.00355.x
Rodriguez del Castillo A, Torres M, Delicado EG, Miras-Portugal MT (1988) Subcellular distribution studies of diadenosine polyphosphates--Ap4A and Ap5A--in bovine adrenal medulla: presence in chromaffin granules. J Neurochem 51(6):1696–1703. https://doi.org/10.1111/j.1471-4159.1988.tb01147.x
Winkler H, Laslop A, Leitner B, Weiss C (1998) The secretory cocktail of adrenergic large dense-core vesicles: the functional role of the chromogranins. Adv Pharmacol 42:257–259. https://doi.org/10.1016/s1054-3589(08)60742-5
Borges R (2013) The ATP or the natural history of neurotransmission. Purinergic Signal 9(1):5–6. https://doi.org/10.1007/s11302-012-9330-7
Pintor J, Torres M, Miras-Portugal MT (1991) Carbachol induced release of diadenosine polyphosphates--Ap4A and Ap5A--from perfused bovine adrenal medulla and isolated chromaffin cells. Life Sci 48(24):2317–2324. https://doi.org/10.1016/0024-3205(91)90268-g
Castro E, Torres M, Miras-Portugal MT, Gonzalez MP (1990) Effect of diadenosine polyphosphates on catecholamine secretion from isolated chromaffin cells. Br J Pharmacol 100(2):360–364. https://doi.org/10.1111/j.1476-5381.1990.tb15809.x
Pintor J, Rotllán P, Torres M, Miras-Portugal MT (1992) Characterization and quantification of diadenosine hexaphosphate in chromaffin cells: granular storage and secretagogue-induced release. Anal Biochem 200(2):296–300. https://doi.org/10.1016/0003-2697(92)90469-n
Castro E, Pintor J, Miras-Portugal MT (1992) Ca(2+)-stores mobilization by diadenosine tetraphosphate, Ap4A, through a putative P2Y purinoceptor in adrenal chromaffin cells. Br J Pharmacol 106(4):833–837. https://doi.org/10.1111/j.1476-5381.1992.tb14421.x
Sen RP, Delicado EG, Castro E, Miras-Portugal MT (1993) Effect of P2Y agonists on adenosine transport in cultured chromaffin cells. J Neurochem 60(2):613–619. https://doi.org/10.1111/j.1471-4159.1993.tb03192.x
Castro E, Tomé AR, Miras-Portugal MT, Rosário LM (1994) Single-cell fura-2 microfluorometry reveals different purinoceptor subtypes coupled to Ca2+ influx and intracellular Ca2+ release in bovine adrenal chromaffin and endothelial cells. Pflugers Arch 426(6):524–533. https://doi.org/10.1007/BF00378530
Castro E, Mateo J, Tomé AR, Barbosa RM, Miras-Portugal MT, Rosário LM Cell-specific purinergic receptors coupled to Ca2+ entry and Ca2+ release from internal stores in adrenal chromaffin cells. Differential sensitivity to UTP and suramin. J Biol Chem 270(10):5098–5106. https://doi.org/10.1074/jbc.270.10.5098
Tomé AR, Castro E, Santos RM, Rosário LM (2007) Selective stimulation of catecholamine release from bovine adrenal chromaffin cells by an ionotropic purinergic receptor sensitive to 2-methylthio ATP. BMC Neurosci 8:41. https://doi.org/10.1186/1471-2202-8-41
Tomé AR, Castro E, Santos RM, Rosário LM (2007) Functional distribution of Ca2 + -coupled P2 purinergic receptors among adrenergic and noradrenergic bovine adrenal chromaffin cells. BMC Neurosci 8:39. https://doi.org/10.1186/1471-2202-8-39
Reichsman F, Santos S, Westhead EW (1995) Two distinct ATP receptors activate calcium entry and internal calcium release in bovine chromaffin cells. J Neurochem 65(5):2080–2086. https://doi.org/10.1046/j.1471-4159.1995.65052080.x
Gandía L, García AG, Morad M (1993) ATP modulation of calcium channels in chromaffin cells. J Physiol 470:55–72. https://doi.org/10.1113/jphysiol.1993.sp019847
Otsuguro K, Ohta T, Ito S, Nakazato Y (1996) Modulation of calcium current by ATP in guinea-pig adrenal chromaffin cells. Pflugers Arch 431(3):402–407. https://doi.org/10.1007/BF02207278
Lim W, Kim SJ, Yan HD, Kim J (1997) Ca2 + -channel-dependent and -independent inhibition of exocytosis by extracellular ATP in voltage-clamped rat adrenal chromaffin cells. Pflugers Arch 435(1):34–42. https://doi.org/10.1007/s004240050481
Ulate G, Scott SR, González J, Gilabert JA, Artalejo AR (2000) Extracellular ATP regulates exocytosis in inhibiting multiple Ca(2+) channel types in bovine chromaffin cells. Pflugers Arch 439(3):304–314. https://doi.org/10.1007/s004249900185
Powell AD, Teschemacher AG, Seward EP (2000) P2Y purinoceptors inhibit exocytosis in adrenal chromaffin cells via modulation of voltage-operated calcium channels. J Neurosci 20(2):606–616. https://doi.org/10.1523/JNEUROSCI.20-02-00606.2000
Harkins AB, Fox AP (2000) Activation of purinergic receptors by ATP inhibits secretion in bovine adrenal chromaffin cells. Brain Res 885(2):231–239. https://doi.org/10.1016/s0006-8993(00)02952-8
Hernández A, Segura-Chama P, Jiménez N, García AG, Hernández-Guijo JM, Hernández-Cruz A (2011) Modulation by endogenously released ATP and opioids of chromaffin cell calcium channels in mouse adrenal slices. Am J Physiol Cell Physiol 300(3):C610–C623. https://doi.org/10.1152/ajpcell.00380.2010
Zhang Q, Liu B, Li Y, Yin L, Younus M, Jiang X, Lin Z, Sun X, Huang R, Liu B, Wu Q, Zhu F, Zhou Z (2020) Regulating quantal size of neurotransmitter release through a GPCR voltage sensor. Proc Natl Acad Sci U S A 117(43):26985–26995. https://doi.org/10.1073/pnas.2005274117
Ennion SJ, Powell AD, Seward EP (2004) Identification of the P2Y(12) receptor in nucleotide inhibition of exocytosis from bovine chromaffin cells. Mol Pharmacol 66(3):601–611. https://doi.org/10.1124/mol.104.000224
Chen XK, Wang LC, Zhou Y, Cai Q, Prakriya M, Duan KL, Sheng ZH, Lingle C, Zhou Z (2005) Activation of GPCRs modulates quantal size in chromaffin cells through G (betagamma) and PKC. Nat Neurosci 8(9):1160–1168. https://doi.org/10.1038/nn1529
Otsuguro K, Asano T, Ohta T, Ito S, Nakazato Y (1995) ATP-evoked membrane current in guinea pig adrenal chromaffin cells. Neurosci Lett 187(3):145–148. https://doi.org/10.1016/0304-3940(95)11359-5
Asano T, Otsuguro K, Ohta T, Sugawara T, Ito S, Nakazato Y (1995) Characteristics of ATP-induced catecholamine secretion from adrenal chromaffin cells of the guinea-pig. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 112(2):101–108. https://doi.org/10.1016/0742-8413(95)02001-2
Liu M, Dunn PM, King BF, Burnstock G (1999) Rat chromaffin cells lack P2X receptors while those of the guinea-pig express a P2X receptor with novel pharmacology. Br J Pharmacol 128(1):61–68. https://doi.org/10.1038/sj.bjp.0702790
Arribas-Blázquez M, Olivos-Oré LA, Barahona MV, Sánchez de la Muela M, Solar V, Jiménez E, Gualix J, McIntosh JM, Ferrer-Montiel A, Miras-Portugal MT, Artalejo AR (2019) Overexpression of P2X3 and P2X7 Receptors and TRPV1 Channels in Adrenomedullary Chromaffin Cells in a Rat Model of Neuropathic Pain. Int J Mol Sci 20(1):155. https://doi.org/10.3390/ijms20010155
Maldifassi MC, Momboisse F, Guerra MJ, Vielma AH, Maripillán J, Báez-Matus X, Flores-Muñoz C, Cádiz B, Schmachtenberg O, Martínez AD, Cárdenas AM (2021) The interplay between α7 nicotinic acetylcholine receptors, pannexin-1 channels and P2X7 receptors elicit exocytosis in chromaffin cells. J Neurochem 157(6):1789–1808. https://doi.org/10.1111/jnc.15186
Acknowledgements
The authors thank Prof. Esmerilda G. Delicado for critical reading of the manuscript.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work was supported by grant PID2019-109155RB-I00 from Ministerio de Ciencia e Innovación of Spain.
Author information
Authors and Affiliations
Contributions
All authors contributed to the article. Antonio R. Artalejo is the corresponding author.
Corresponding author
Ethics declarations
Conflicts of interest
Antonio R. Artalejo declares that there is no conflict of interest. Marina Arribas-Blázquez declares that there is no conflict of interest. María Victoria Barahona declares that there is no conflict of interest. Celia Llorente-Sáez declares that there is no conflict of interest. Luis Alcides Olivos-Oré declares that there is no conflict of interest.
Ethics approval
This article does not contain any studies with human participants or animals performed by the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Article for the special thematic issue on “Small molecules and biologics for future purinergic therapeutics. A Tribute to Maria Teresa Miras Portugal”
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Artalejo, A.R., Arribas-Blázquez, M., Barahona, M.V. et al. María Teresa Miras Portugal: a pioneer in the study of purinoceptors in chromaffin cells. Purinergic Signalling 20, 109–113 (2024). https://doi.org/10.1007/s11302-023-09934-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-023-09934-1