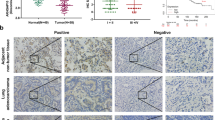
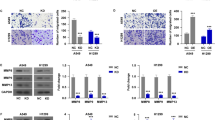
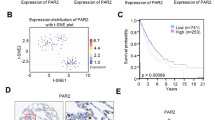
Abstract
It has been demonstrated that the ATP-gated ion channel P2X7 receptor is involved in tumor progression and plays an important role in regulating tumor cell growth, invasion, migration and angiogenesis. However, P2X7 receptors have been relatively poorly studied in non-small cell lung cancer (NSCLC) cells. Therefore, the aim of this study was to investigate the effects of P2X7 receptor on A549 cells (NSCLC cell line) migration and invasion and to reveal the molecular mechanisms mediated by it. We detected the expression and function of P2X7 receptor in A549 cells. The effects and mechanisms of P2X7 receptor on A549 cells migration, invasion, and epithelial-mesenchymal transition were detected in vitro and in vivo. The results showed P2X7 receptor expressed by A549 cells had ion channel and macropore formation function. In addition, activation of P2X7 receptor by adenosine triphosphate (ATP) or 2′(3′)-O-(4-Benzoylbenzoyl)-adenosine-5'-triphosphate (BzATP) promoted Epithelial-mesenchymal transition (EMT), migration and invasion of A549 cells, which was attenuated by treatment of cells with P2X7 receptor antagonist A438079 and Oxidized ATP. Furthermore, activation of P2X7 receptor increased phosphorylated protein kinase B (p-Akt) levels, and the phosphatidylinositol-tris–phosphate kinase 3 (PI3K)/protein kinase B (Akt) inhibitor LY294002 blocked migration and invasion of A549 cells induced by ATP or BzATP. At the same time, in vivo results showed that P2X7 receptor could also promote EMT and PI3K/Akt expression in transplanted tumors. Our study indicated that P2X7 receptor promotes A549 cells migration and invasion through the PI3K/Akt signaling pathway, suggesting that P2X7 receptor may be a potential therapeutic target for NSCLC.





Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the article.
References
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70:7–30. https://doi.org/10.3322/caac.21590
Nasim F, Sabath BF, Eapen GA (2019) Lung cancer. Med Clin North Am 103:463–473. https://doi.org/10.1016/j.mcna.2018.12.006
Elias R, Morales J, Presley C (2017) Checkpoint inhibitors for non-small cell lung cancer among older adults. Curr Oncol Rep 19:62. https://doi.org/10.1007/s11912-017-0619-0
Akram A, Khalil S, Halim SA, Younas H, Iqbal S, Mehar S (2018) Therapeutic Uses of HSP90 Inhibitors in Non-Small Cell Lung Carcinoma (NSCLC). Curr Drug Metab 19:335–341. https://doi.org/10.2174/1389200219666180307122441
Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL (2019) Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 69:363–385. https://doi.org/10.3322/caac.21565
Huang MY, Jiang XM, Wang BL, Sun Y, Lu JJ (2021) Combination therapy with PD-1/PD-L1 blockade in non-small cell lung cancer: strategies and mechanisms. Pharmacol Ther 219:107694. https://doi.org/10.1016/j.pharmthera.2020.107694
Wood SL, Pernemalm M, Crosbie PA, Whetton AD (2014) The role of the tumor-microenvironment in lung cancer-metastasis and its relationship to potential therapeutic targets. Cancer Treat Rev 40:558–566. https://doi.org/10.1016/j.ctrv.2013.10.001
Herbst RS, Morgensztern D, Boshoff C (2018) The biology and management of non-small cell lung cancer. Nature 553:446–454. https://doi.org/10.1038/nature25183
Imyanitov EN, Iyevleva AG, Levchenko EV (2021) Molecular testing and targeted therapy for non-small cell lung cancer: Current status and perspectives. Crit Rev Oncol Hematol 157:103194. https://doi.org/10.1016/j.critrevonc.2020.103194
Naylor EC, Desani JK, Chung PK (2016) Targeted therapy and immunotherapy for lung cancer. Surg Oncol Clin N Am 25:601–609. https://doi.org/10.1016/j.soc.2016.02.011
Nagasaka M, Gadgeel SM (2018) Role of chemotherapy and targeted therapy in early-stage non-small cell lung cancer. Expert Rev Anticancer Ther 18:63–70. https://doi.org/10.1080/14737140.2018.1409624
Arbour KC, Riely GJ (2019) Systemic therapy for locally advanced and metastatic non-small cell lung cancer: a review. JAMA 322:764–774. https://doi.org/10.1001/jama.2019.11058
Burnstock G (1997) The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology 36:1127–1139. https://doi.org/10.1016/s0028-3908(97)00125-1
Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F (2008) Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS One 3:e2599. https://doi.org/10.1371/journal.pone.0002599
Di Virgilio F, Falzoni S, Giuliani AL, Adinolfi E (2016) P2 receptors in cancer progression and metastatic spreading. Curr Opin Pharmacol 29:17–25. https://doi.org/10.1016/j.coph.2016.05.001
Burnstock G (2009) Purinergic signalling: past, present and future. Braz J Med Biol Res 42:3–8. https://doi.org/10.1590/s0100-879x2008005000037
Furini F, Giuliani AL, Parlati ME, Govoni M, Di Virgilio F, Bortoluzzi A (2019) P2X7 receptor expression in patients with serositis related to systemic lupus erythematosus. Front Pharmacol 10:435. https://doi.org/10.3389/fphar.2019.00435
Salaro E, Rambaldi A, Falzoni S, Amoroso FS, Franceschini A, Sarti AC, Bonora M, Cavazzini F, Rigolin GM, Ciccone M, Audrito V, Deaglio S, Pelegrin P, Pinton P, Cuneo A, Di Virgilio F (2016) Involvement of the P2X7-NLRP3 axis in leukemic cell proliferation and death. Sci Rep 6:26280. https://doi.org/10.1038/srep26280
Sluyter R (2017) The P2X7 receptor. Adv Exp Med Biol 1051:17–53. https://doi.org/10.1007/5584_2017_59
Zou J, Vetreno RP, Crews FT (2012) ATP-P2X7 receptor signaling controls basal and TNFα-stimulated glial cell proliferation. Glia 60:661–673. https://doi.org/10.1002/glia.22302
Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S (2017) The P2X7 receptor in infection and inflammation. Immunity 47:15–31. https://doi.org/10.1016/j.immuni.2017.06.020
Baroja-Mazo A, Barberà-Cremades M, Pelegrín P (2013) The participation of plasma membrane hemichannels to purinergic signaling. Biochim Biophys Acta 1828:79–93. https://doi.org/10.1016/j.bbamem.2012.01.002
Browne LE, Compan V, Bragg L, North RA (2013) P2X7 receptor channels allow direct permeation of nanometer-sized dyes. J Neurosci 33:3557–3566. https://doi.org/10.1523/JNEUROSCI.2235-12.2013
Jiang R, Taly A, Grutter T (2013) Trends Biochem Sci 38:20–29. https://doi.org/10.1016/j.tibs.2012.10.006
Volonté C, Apolloni S, Skaper SD, Burnstock G (2012) P2X7 receptors: channels, pores and more. CNS Neurol Disord Drug Targets 11:705–721. https://doi.org/10.2174/187152712803581137
Zhang WJ, Hu CG, Zhu ZM, Luo HL (2020) Effect of P2X7 receptor on tumorigenesis and its pharmacological properties. Biomed Pharmacother 125:109844. https://doi.org/10.1016/j.biopha.2020.109844
Di Virgilio F, Chiozzi P, Falzoni S, Ferrari D, Sanz JM, Venketaraman V, Baricordi OR (1998) Cytolytic P2X purinoceptors. Cell Death Differ 5:191–199. https://doi.org/10.1038/sj.cdd.4400341
Souza CO, Santoro GF, Figliuolo VR, Nanini HF, de Souza HS, Castelo-Branco MT, Abalo AA, Paiva MM, Coutinho CM, Coutinho-Silva R (2012) Extracellular ATP induces cell death in human intestinal epithelial cells. Biochim Biophys Acta 1820:1867–1878. https://doi.org/10.1016/j.bbagen.2012.08.013
Salvestrini V, Orecchioni S, Talarico G, Reggiani F, Mazzetti C, Bertolini F, Orioli E, Adinolfi E, Di Virgilio F, Pezzi A, Cavo M, Lemoli RM, Curti A (2017) Extracellular ATP induces apoptosis through P2X7R activation in acute myeloid leukemia cells but not in normal hematopoietic stem cells. Oncotarget 8:5895–5908. https://doi.org/10.18632/oncotarget.13927
Tamajusuku AS, Villodre ES, Paulus R, Coutinho-Silva R, Battasstini AM, Wink MR, Lenz G (2010) Characterization of ATP-induced cell death in the GL261 mouse glioma. J Cell Biochem 109:983–991. https://doi.org/10.1002/jcb.22478
Choi JH, Ji YG, Ko JJ, Cho HJ, Lee DH (2018) Activating P2X7 receptors increases proliferation of human pancreatic cancer cells via ERK1/2 and JNK. Pancreas 47:643–651. https://doi.org/10.1097/MPA.0000000000001055
Raffaghello L, Chiozzi P, Falzoni S, Di Virgilio F, Pistoia V (2006) The P2X7 receptor sustains the growth of human neuroblastoma cells through a substance P-dependent mechanism. Cancer Res 66:907–914. https://doi.org/10.1158/0008-5472.CAN-05-3185
Adinolfi E, Raffaghello L, Giuliani AL, Cavazzini L, Capece M, Chiozzi P, Bianchi G, Kroemer G, Pistoia V, Di Virgilio F (2012) Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res 72:2957–2969. https://doi.org/10.1158/0008-5472.CAN-11-1947
Jelassi B, Anchelin M, Chamouton J, Cayuela ML, Clarysse L, Li J, Goré J, Jiang LH, Roger S (2013) Anthraquinone emodin inhibits human cancer cell invasiveness by antagonizing P2X7 receptors. Carcinogenesis 34:1487–1496. https://doi.org/10.1093/carcin/bgt099
Qiu Y, Li WH, Zhang HQ, Liu Y, Tian XX, Fang WG (2014) P2X7 mediates ATP-driven invasiveness in prostate cancer cells. PLoS One 9:e114371. https://doi.org/10.1371/journal.pone.0114371
Takai E, Tsukimoto M, Harada H, Kojima S (2014) Autocrine signaling via release of ATP and activation of P2X7 receptor influences motile activity of human lung cancer cells. Purinergic Signal 10:487–497. https://doi.org/10.1007/s11302-014-9411-x
Takai E, Tsukimoto M, Harada H, Sawada K, Moriyama Y, Kojima S (2012) Autocrine regulation of TGF-β1-induced cell migration by exocytosis of ATP and activation of P2 receptors in human lung cancer cells. J Cell Sci 125:5051–5060. https://doi.org/10.1242/jcs.104976
Schmid S, Kübler M, Korcan Ayata C, Lazar Z, Haager B, Hoßfeld M, Meyer A, Cicko S, Elze M, Wiesemann S, Zissel G, Passlick B, Idzko M (2015) Altered purinergic signaling in the tumor associated immunologic microenvironment in metastasized non-small-cell lung cancer. Lung Cancer 90:516–521. https://doi.org/10.1016/j.lungcan.2015.10.005
Zanini D, Manfredi LH, Pelinson LP, Pimentel VC, Cardoso AM, Carmo Araújo Gonçalves VD, Santos CBD, Gutierres JM, Morsch VM, Leal DBR, Schetinger MRC (2019) ADA activity is decreased in lymphocytes from patients with advanced stage of lung cancer. Med Oncol 36:78. https://doi.org/10.1007/s12032-019-1301-1
Boldrini L, Giordano M, Alì G, Melfi F, Romano G, Lucchi M, Fontanini G (2015) P2X7 mRNA expression in non-small cell lung cancer: MicroRNA regulation and prognostic value. Oncol Lett 9:449–453. https://doi.org/10.3892/ol.2014.2620
Singh M, Yelle N, Venugopal C, Singh SK (2018) EMT: Mechanisms and therapeutic implications. Pharmacol Ther 182:80–94. https://doi.org/10.1016/j.pharmthera.2017.08.009
Derynck R, Weinberg RA (2019) EMT and cancer: more than meets the eye. Dev Cell 49:313–316. https://doi.org/10.1016/j.devcel.2019.04.026
Hashioka S, Wang YF, Little JP, Choi HB, Klegeris A, McGeer PL, McLarnon JG (2014) Purinergic responses of calcium-dependent signaling pathways in cultured adult human astrocytes. BMC Neurosci 15:18. https://doi.org/10.1186/1471-2202-15-18
Kaushal V, Dye R, Pakavathkumar P, Foveau B, Flores J, Hyman B, Ghetti B, Koller BH, LeBlanc AC (2015) Neuronal NLRP1 inflammasome activation of Caspase-1 coordinately regulates inflammatory interleukin-1-beta production and axonal degeneration-associated Caspase-6 activation. Cell Death Differ 22:1676–1686. https://doi.org/10.1038/cdd.2015.16
Xia J, Yu X, Tang L, Li G, He T (2015) P2X7 receptor stimulates breast cancer cell invasion and migration via the AKT pathway. Oncol Rep 34:103–110. https://doi.org/10.3892/or.2015.3979
Tang Y, Zhao R, Qiao C, Li X, Bai X, Peng X (2022) P2X7R promotes migration and invasion of Lewis lung cancer cells by activating the AKT signaling pathway. Nan Fang Yi Ke Da Xue Xue Bao 42:1495–1502. https://doi.org/10.12122/j.issn.1673-4254.2022.10.08
Zhang WJ, Hu CG, Luo HL, Zhu ZM (2020) Activation of P2×7 receptor promotes the invasion and migration of colon cancer cells via the STAT3 signaling. Front Cell Dev Biol 8:586555. https://doi.org/10.3389/fcell.2020.586555
Boldrini L, Giordano M, Alì G, Servadio A, Pelliccioni S, Niccoli C, Mussi A, Fontanini G (2014) P2X7 protein expression and polymorphism in non-small cell lung cancer (NSCLC). J Negat Results Biomed 13:16. https://doi.org/10.1186/1477-5751-13-16
Zhang Y, Ding J, Wang L (2019) The role of P2X7 receptor in prognosis and metastasis of colorectal cancer. Adv Med Sci 64:388–394. https://doi.org/10.1016/j.advms.2019.05.002
Tan C, Han LI, Zou L, Luo C, Liu A, Sheng X, Xi D (2015) Expression of P2X7R in breast cancer tissue and the induction of apoptosis by the gene-specific shRNA in MCF-7 cells. Exp Ther Med 10:1472–1478. https://doi.org/10.3892/etm.2015.2705
Lili W, Yun L, Tingran W, Xia W, Yanlei S (2019) P2RX7 functions as a putative biomarker of gastric cancer and contributes to worse prognosis. Exp Biol Med (Maywood) 244:734–742. https://doi.org/10.1177/1535370219846492
Giannuzzo A, Saccomano M, Napp J, Ellegaard M, Alves F, Novak I (2016) Targeting of the P2X7 receptor in pancreatic cancer and stellate cells. Int J Cancer 139:2540–2552. https://doi.org/10.1002/ijc.30380
Solini A, Cuccato S, Ferrari D, Santini E, Gulinelli S, Callegari MG, Dardano A, Faviana P, Madec S, Di Virgilio F, Monzani F (2008) Increased P2X7 receptor expression and function in thyroid papillary cancer: a new potential marker of the disease? Endocrinology 149:389–396. https://doi.org/10.1210/en.2007-1223
Li X, Zhou L, Feng YH, Abdul-Karim FW, Gorodeski GI (2006) The P2X7 receptor: a novel biomarker of uterine epithelial cancers. Cancer Epidemiol Biomarkers Prev 15:1906–1913. https://doi.org/10.1158/1055-9965.EPI-06-0407
Calik I, Calik M, Sarikaya B, Ozercan IH, Arslan R, Artas G, Dagli AF (2020) P2X7 receptor as an independent prognostic indicator in gastric cancer. Bosn J Basic Med Sci 20:188–196. https://doi.org/10.17305/bjbms.2020.4620
Qian F, Xiao J, Hu B, Sun N, Yin W, Zhu J (2017) High expression of P2X7R is an independent postoperative indicator of poor prognosis in colorectal cancer. Hum Pathol 64:61–68. https://doi.org/10.1016/j.humpath.2017.03.019
Cao F, Hu LQ, Yao SR, Hu Y, Wang DG, Fan YG, Pan GX, Tao SS, Zhang Q, Pan HF, Wu GC (2019) P2X7 receptor: A potential therapeutic target for autoimmune diseases. Autoimmun Rev 18:767–777. https://doi.org/10.1016/j.autrev.2019.06.009
Gilbert SM, Oliphant CJ, Hassan S, Peille AL, Bronsert P, Falzoni S, Di Virgilio F, McNulty S, Lara R (2019) ATP in the tumour microenvironment drives expression of nfP2X7, a key mediator of cancer cell survival. Oncogene 38:194–208. https://doi.org/10.1038/s41388-018-0426-6
Giannuzzo A, Pedersen SF, Novak I (2015) The P2X7 receptor regulates cell survival, migration and invasion of pancreatic ductal adenocarcinoma cells. Mol Cancer 14:203. https://doi.org/10.1186/s12943-015-0472-4
Wei W, Ryu JK, Choi HB, McLarnon JG (2008) Expression and function of the P2X(7) receptor in rat C6 glioma cells. Cancer Lett 260:79–87. https://doi.org/10.1016/j.canlet.2007.10.025
Fang J, Chen X, Zhang L, Chen J, Liang Y, Li X, Xiang J, Wang L, Guo G, Zhang B, Zhang W (2013) P2X7R suppression promotes glioma growth through epidermal growth factor receptor signal pathway. Int J Biochem Cell Biol 45:1109–1120. https://doi.org/10.1016/j.biocel.2013.03.005
Hattori F, Ohshima Y, Seki S, Tsukimoto M, Sato M, Takenouchi T, Suzuki A, Takai E, Kitani H, Harada H, Kojima S (2012) Feasibility study of B16 melanoma therapy using oxidized ATP to target purinergic receptor P2X7. Eur J Pharmacol 695:20–26. https://doi.org/10.1016/j.ejphar.2012.09.001
Tafani M, Schito L, Pellegrini L, Villanova L, Marfe G, Anwar T, Rosa R, Indelicato M, Fini M, Pucci B, Russo MA (2011) Hypoxia-increased RAGE and P2X7R expression regulates tumor cell invasion through phosphorylation of Erk1/2 and Akt and nuclear translocation of NF-{kappa}B. Carcinogenesis 32:1167–1175. https://doi.org/10.1093/carcin/bgr101
Zhang Y, Cheng H, Li W, Wu H, Yang Y (2019) Highly-expressed P2X7 receptor promotes growth and metastasis of human HOS/MNNG osteosarcoma cells via PI3K/Akt/GSK3β/β-catenin and mTOR/HIF1α/VEGF signaling. Int J Cancer 145:1068–1082. https://doi.org/10.1002/ijc.32207
Ji Z, Xie Y, Guan Y, Zhang Y, Cho KS, Ji M, You Y (2018) Involvement of P2X7 Receptor in Proliferation and Migration of Human Glioma Cells. Biomed Res Int 2018:8591397. https://doi.org/10.1155/2018/8591397
Zhang WJ, Luo C, Huang C, Pu FQ, Zhu JF, Zhu ZM (2021) PI3K/Akt/GSK-3β signal pathway is involved in P2X7 receptor-induced proliferation and EMT of colorectal cancer cells. Eur J Pharmacol 899:174041. https://doi.org/10.1016/j.ejphar.2021.174041
Jelassi B, Chantôme A, Alcaraz-Pérez F, Baroja-Mazo A, Cayuela ML, Pelegrin P, Surprenant A, Roger S (2011) P2X(7) receptor activation enhances SK3 channels- and cystein cathepsin-dependent cancer cells invasiveness. Oncogene 30:2108–2122. https://doi.org/10.1038/onc.2010.593
Sharma S, Kalra H, Akundi RS (2020) Extracellular ATP mediates cancer cell migration and invasion through increased expression of cyclooxygenase 2. Front Pharmacol 11:617211. https://doi.org/10.3389/fphar.2020.617211
Shi K, Queiroz KC, Stap J, Richel DJ, Spek CA (2013) Protease-activated receptor-2 induces migration of pancreatic cancer cells in an extracellular ATP-dependent manner. J Thromb Haemost 11:1892–1902. https://doi.org/10.1111/jth.12361
Fang WG, Tian XX (2017) Identification of a new pro-invasion factor in tumor microenvironment: progress in function and mechanism of extracellular ATP. Beijing Da Xue Xue Bao Yi Xue Ban 49:188–195
Schneider G, Glaser T, Lameu C, Abdelbaset-Ismail A, Sellers ZP, Moniuszko M, Ulrich H, Ratajczak MZ (2015) Extracellular nucleotides as novel, underappreciated pro-metastatic factors that stimulate purinergic signaling in human lung cancer cells. Mol Cancer 14:201. https://doi.org/10.1186/s12943-015-0469-z
Cao Y, Wang X, Li Y, Evers M, Zhang H, Chen X (2019) Extracellular and macropinocytosis internalized ATP work together to induce epithelial-mesenchymal transition and other early metastatic activities in lung cancer. Cancer Cell Int 19:254. https://doi.org/10.1186/s12935-019-0973-0
He J (2017) Knocking down MiR-15a expression promotes the occurrence and development and induces the EMT of NSCLC cells in vitro. Saudi J Biol Sci 24:1859–1865. https://doi.org/10.1016/j.sjbs.2017.11.028
Tang Z, Ding Y, Shen Q, Zhang C, Li J, Nazar M, Wang Y, Zhou X, Huang J (2019) KIAA1199 promotes invasion and migration in non-small-cell lung cancer (NSCLC) via PI3K-Akt mediated EMT. J Mol Med (Berl) 97:127–140. https://doi.org/10.1007/s00109-018-1721-y
Panenka W, Jijon H, Herx LM, Armstrong JN, Feighan D, Wei T, Yong VW, Ransohoff RM, MacVicar BA (2001) P2X7-like receptor activation in astrocytes increases chemokine monocyte chemoattractant protein-1 expression via mitogen-activated protein kinase. J Neurosci 21:7135–7142. https://doi.org/10.1523/JNEUROSCI.21-18-07135.2001
Jacques-Silva MC, Rodnight R, Lenz G, Liao Z, Kong Q, Tran M, Kang Y, Gonzalez FA, Weisman GA, Neary JT (2004) P2X7 receptors stimulate AKT phosphorylation in astrocytes. Br J Pharmacol 141:1106–1117. https://doi.org/10.1038/sj.bjp.0705685
Humphreys BD, Rice J, Kertesy SB, Dubyak GR (2000) Stress-activated protein kinase/JNK activation and apoptotic induction by the macrophage P2X7 nucleotide receptor. J Biol Chem 275:26792–26798. https://doi.org/10.1074/jbc.M002770200
Altomare DA, Testa JR (2005) Perturbations of the AKT signaling pathway in human cancer. Oncogene 24:7455–7464. https://doi.org/10.1038/sj.onc.1209085
Gómez-Villafuertes R, García-Huerta P, Díaz-Hernández JI, Miras-Portugal MT (2015) PI3K/Akt signaling pathway triggers P2X7 receptor expression as a pro-survival factor of neuroblastoma cells under limiting growth conditions. Sci Rep 5:18417. https://doi.org/10.1038/srep18417
Vázquez-Cuevas FG, Martínez-Ramírez AS, Robles-Martínez L, Garay E, García-Carrancá A, Pérez-Montiel D, Castañeda-García C, Arellano RO (2014) Paracrine stimulation of P2X7 receptor by ATP activates a proliferative pathway in ovarian carcinoma cells. J Cell Biochem 115:1955–1966. https://doi.org/10.1002/jcb.24867
Bian S, Sun X, Bai A, Zhang C, Li L, Enjyoji K, Junger WG, Robson SC, Wu Y (2013) P2X7 integrates PI3K/AKT and AMPK-PRAS40-mTOR signaling pathways to mediate tumor cell death. PLoS One 8:e60184. https://doi.org/10.1371/journal.pone.0060184
Pérez-Ramírez C, Cañadas-Garre M, Molina MÁ, Faus-Dáder MJ, Calleja-Hernández MÁ (2015) PTEN and PI3K/AKT in non-small-cell lung cancer. Pharmacogenomics 16:1843–1862. https://doi.org/10.2217/pgs.15.12
Vultaggio-Poma V, Sarti AC, Di Virgilio F (2020) Extracellular ATP: a feasible target for cancer therapy. Cells 9:2496. https://doi.org/10.3390/cells9112496
Funding
This work was supported by Shandong Provincial Natural Science Foundation (no. ZR2021MH306) and the National Natural Science Foundation of China (no. 81770915).
Author information
Authors and Affiliations
Contributions
RLZ designed and supervised the study. RLZ, XB, QQL, XXP, XYL, CCQ and YQT performed the experiments. RLZ, XB, QQL, XXP, XYL, CCQ and YQT analyzed the results. RLZ, XB, QQL, XXP drafted the manuscript. RLZ, XB, QQL, XXP revised the manuscript. All authors approved the manuscript submitted.
Corresponding author
Ethics declarations
Ethics approval
All animal experiments were approved by the Animal Care Committee of Weifang Medical University (No 2021SDL124) and performed according to the guidelines of the National Institutes of Health.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Informed consent
Not applicable.
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Conflicts of interest
The authors declare that they have no potential conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bai, X., Li, Q., Peng, X. et al. P2X7 receptor promotes migration and invasion of non-small cell lung cancer A549 cells through the PI3K/Akt pathways. Purinergic Signalling 19, 685–697 (2023). https://doi.org/10.1007/s11302-023-09928-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-023-09928-z