Abstract
The basic research indicated that microglial P2Y12 receptors (P2Y12Rs) are involved in the pathophysiology of epilepsy through regulated microglial-neuronal interactions, aberrant neurogenesis, or immature neuronal projections. However, whether the clinic case of epilepsy would be associated with P2Y12 receptor gene polymorphisms is presented with few data. In our study, a total of 176 patients with epilepsy and 50 healthy controls were enrolled. Two single-nucleotide polymorphisms, namely rs1491974 and rs6798347, were selected for analysis. The results revealed that carriers of the G allele of rs1491974 G>A or rs6798347 G>A may be associated with an increased risk of epilepsy (OR = 0.576, 95% CI = 0.368–0.901, p = 0.015; OR = 0.603, 95% CI = 0.367–0.988, p = 0.043). Interestingly, we found that the rs1491974 G>A genotype and allele frequencies have only a significant difference in female instead of male case (p = 0.004 for genotype; p = 0.001 for allele). The subgroup analysis demonstrated that individuals with the rs1491974 G>A genotype might have more frequent seizure (OR = 0.476, 95% CI = 0.255–0.890; p = 0.019). These data implied that both rs1491974 and rs6798347 polymorphisms of P2Y12R would be able to play import roles in epilepsy susceptibility, whereas the rs1491974 polymorphism may be specifically related to seizure frequency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy is one of the most common neurological disorders which affects over 70 million people globally and imposes a considerable socio-economic burden [1,2,3]. The etiology of epilepsy is diverse and remains elusive [4]. Among various factors, genetic mutations, such as single-nucleotide polymorphisms (SNPs), are a common cause of epilepsy and are generally associated with ion channels, neuronal receptors, transcription factors, and enzymes [5,6,7,8. Accumulating evidence has shown that purinergic signaling SNPs, including adenosine kinase SNPs, adenosine A1 receptor SNPs, and adenosine A2A receptor SNPs, are implicated in the pathogenesis mechanism of epilepsy [9,10,11]. However, purinergic signaling is a big family. It includes purines (ATP, ADP, AMP, adenosine), enzymes (CD39, CD73), and purinergic receptors (four P1 receptors, seven P2X receptors, and eight P2Y receptors). Moreover, purinergic signaling has been recognized as promising targets for the treatment of various central nervous system (CNS) diseases [12,13,14,15,16]. The role of these purinergic signaling SNPs in epilepsy, especially P2Y12 receptor (P2Y12R), has not been investigated yet.
The P2Y12 receptor is part of the metabotropic (P2Y) receptor family with exclusive expression in the microglia of the CNS [17,18], and it is essential for brain homeostasis including synaptic plasticity [19], vascular repair [20], and chemotaxis and motility of microglia [21,22]. Several studies revealed the P2Y12R plays a pivotal role in the pathophysiology of several CNS disorders, including seizures [23,24,25,26,27]. Eyo and colleagues reported that seizure outcome worsened in P2Y12R knockout mice after kainic acid injection—suggesting a neuroprotective role for microglial P2Y12R in epilepsy [24]. This is probably because the ATP released by hyperactive neurons increases neurons-microglia contact via P2Y12R during the seizure-onset phase and in consequence exerts a suppression of neuronal activity via A1 receptors [28,29]. Further study indicated that a P2Y12R-dependent mechanism in microglia promoted aberrant neurogenesis and increased immature neuronal projections following seizures, which contributed to the development of epilepsy [26]. If P2Y12R is proved to impact on microglia function during epilepsy in basic research, could its single-nucleotide polymorphism be involved in the etiology of epilepsy?
Previous studies demonstrated that the P2Y12R rs1491974 might be related to moderate residual platelet reactivity in coronary artery disease, while the P2Y12R rs6798347 could be associated with ADP-induced platelet aggregation [30,31]. However, the relationship between these two P2Y12R SNPs and epilepsy is unclear. Therefore, our study aimed to investigate the association between P2Y12R gene polymorphisms (rs1491974 and rs6798347) and epilepsy.
Patients and methods
Study participants
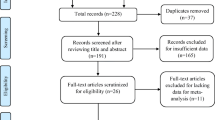
As a case-control study, 200 patients with epilepsy (PWEs) and 50 healthy participants were recruited at the Sichuan Academy of Medical Science and Sichuan Provincial People’s Hospital in China between August 2020 and August 2021. All study protocols were approved by the Ethics Committee of the Sichuan Academy of Medical Science and Sichuan Provincial People’s Hospital. Written informed consent was obtained from either the participants or their guardians. PWEs were diagnosed according to the 2014 International League Against Epilepsy criteria [32]. Those with a history of pseudo-epileptic seizures, as well as those with impaired hepatic and/or renal function, were excluded. The clinical data of patients were collected, including gender, age, disease diagnosis, seizure onset frequency, epilepsy onset, medical history, and imaging examination. Individuals missing the above-mentioned clinical data were excluded from the study. The healthy controls were neurologically normal and had no personal or family history of epilepsy.
DNA extraction and genotyping
A 5-mL sample of anti-coagulant peripheral blood was taken from each participant. Genomic DNA was extracted from whole blood samples using a Qiagen kit (Qiagen, Hilden, Germany) according to the manufacturer’s recommendations and stored at –80 °C until further use. All SNPs were genotyped using the MassARRAY platform (Agenda Bioscience, San Diego, CA, USA) at CapitalBio (Beijing, China). The primers for PCR amplification and extension were designed using the MassARRAY Assay Design v4.0 software. The PCR cycle program as well as shrimp alkaline phosphatase digestion and extension was performed according to the manufacturer’s protocol. Extension products were desalted and detected using matrix-assisted laser desorption ionization time-of-flight. Finally, the data was processed with TYPER v4.0 software (Agena Bioscience, San Diego, CA, USA).
Statistical analysis
All statistical analyses were conducted with SPSS v26.0 software (Chicago, IL, USA). Categorical variables of baseline characteristics were performed as proportions and continuous variables as medians with interquartile ranges. Differences in the demographic characteristics between the two groups were analyzed by the non-parametric independent-samples Wilcoxon signed-rank test for continuous variables and the chi-square test for categorical data. The chi-square test was used to assess the deviation from Hardy–Weinberg equilibrium. The chi-square statistics or Fisher’s exact test was used to compare the statistical differences in genotype distributions and allele frequencies between cases and controls. The odds ratio (OR) was calculated with 95% confidence intervals (CIs). Statistical significance was defined as two-tailed p < 0.05.
Results
Clinical characteristics of the study population
A total of 200 PWEs participated in the study, with 192 of them satisfactorily genotyped for both SNPs. A total of 16 participants were removed from the study due to a lack of clinical data. Therefore, our study included 176 PWEs (85 males, 91 females; median age: 29 years) and 50 healthy controls (22 males, 28 females; median age: 26 years). There was no statistically significant difference between epileptic patients and healthy controls in terms of gender. Table 1 demonstrates the demographic and clinical characteristics of the study population.
Associations of the P2Y12R gene polymorphisms with epilepsy
Table 2 shows the genotypes or alleles of the two SNPs (rs1491974 and rs6798347) in PWEs and controls. Subsequently, we stratified the groups by gender, neuroimaging, epileptic seizure frequency, and treatment response (Tables 3, 4, 5, and 6). Our results demonstrated that the frequency of the rs1491974 G allele was significantly higher among all patients than in healthy controls (OR = 0.576, 95% CI = 0.368–0.901, p = 0.015 for A vs. G). We also found the distribution of the G allele of epileptic patients with negative intracranial imaging was significantly higher than that of the healthy individuals (OR = 0.600, 95% CI = 0.369–0.975, p = 0.038 for A vs. G). These results illustrated those individuals with the G allele of rs1491974 G>A might have higher risks for epilepsy. After separating the groups by gender, the differences appeared to be limited to healthy controls and female patients (p = 0.004 for genotype; p = 0.001 for allele). In female patients, we found the GG genotype frequency was markedly higher than that of the controls (OR = 3.450, 95% CI = 1.204–9.883, p = 0.017 for GG vs. AA/AG), indicating that the GG genotype of P2Y12R rs1491974 may be closely related to epilepsy susceptibility in females. Subgroup analyses were also conducted stratified for epileptic seizure frequency. We found the homozygous AA and GG genotypes were associated with a lower risk of frequent seizures for patients, while the heterozygous AG genotype was related to a higher risk (OR = 0.476, 95% CI = 0.255–0.890; p = 0.019 for AA/GG vs. GG). Additionally, we did not detect a significant association between rs1491974 and rs6798347 with the P2Y12R gene and treatment response.
For the P2Y12R rs6798347 G>A polymorphism, the frequency of the G allele was substantially greater in all patients than in the healthy controls (OR = 0.603, 95% CI = 0.367–0.988, p = 0.043 for A vs. G). Comparing PWEs with negative intracranial imaging and healthy controls, there were no variations in allelic or genotypic distribution. In addition, after grouping by gender, epileptic seizure frequency, and treatment response, we discovered that there were no significant differences between PWEs and controls.
Discussion
The results of the present study showed a significant difference in the G allele frequency of P2Y12R rs1491974 and rs6798347 polymorphisms between PWEs and healthy participants, indicating that P2Y12R genetic variability might be associated with epilepsy. Consistent with our result, animal studies have shown that P2Y12R-deficient mice had exacerbated behavioral seizures after intraperitoneal kainic acid injection and the percentage of mice showing seizures increased by inactivating the P2Y12R gene [24],[26],[33]. From these findings, P2Y12R was presented to play a role in epilepsy.
The P2Y12 receptor was originally thought to be exclusively expressed on platelets [34]. Thus, a great deal of previous research about P2Y12R gene polymorphisms focused on platelet aggregation or pharmacological response to anti-platelet drugs [35,36,37,38]. Nevertheless, recent studies demonstrated that the P2Y12R is expressed and functional in microglial cells [39,40],Gómez et al., 2021,[41]. Microglial P2Y12R detects the synaptic release of ATP after neuronal activity and controls chemotaxis and motility of microglia, which are involved in microglia-mediated suppression of neuronal activities [14, 15, 28, 29, 42]. As mentioned, the findings of Badimon et al. in P2Y12R-inactivated mice suggest microglial P2Y12R may have a hyperactivity-limiting role in epilepsy [33]. However, in contrast, another research group found that P2Y12 receptor may play hyperactivity-promoting roles in epileptogenesis after the initial seizure, since a P2Y12R deficiency in mice limits the extent of neurogenesis and sprouting that are thought to promote spontaneous recurring seizures [26]. Moreover, the extracellular ADP promoted the activation of NLRP3 inflammasomes and the release of IL-1β and IL-6 via the P2Y12 receptor [43]. These inflammatory cytokines could promote neurogenesis and the excitability of neurons, which may lead to the development of epilepsy [44,45]. Together, P2Y12R SNPs may impact on microglia function during epilepsy. To prove our hypothesis, further studies should be performed to verify the association of microglia and P2Y12R SNPs.
Several epidemiologic studies have indicated that epilepsy was more common in males than in females [46,47,48,49]. Interestingly, our findings indicated the G allele or GG genotype of rs1491974 (G>A) was more predominant in female patients with epilepsy than in their control counterparts, whereas no significant differences were shown in males. A potential explanation for the disparity may involve the differences in endogenous sex hormones, such as androgen, estrogen, and progesterone, as well as their metabolites, which play a vital role in brain network construction and neuro-immune system activity [50]. This agrees with the findings of Wang et al. [51] and [52], who discovered that gender-specific incidence was higher for male partial seizures than for females in NLRP1 SNPs (rs878329, G>C) and NRG1 SNPs (rs35753505, T>C). We also observed an increased seizure frequency in individuals with the AG genotype of P2Y12R rs1491974. Overall, our findings suggest that P2Y12R gene variants influence some characteristics of expression in epilepsy patients. In addition, though our results suggest that P2Y12R gene polymorphisms do not correlate with the response to antiepileptic drugs, this still requires further investigation.
Our study is not without limitations. First, we only analyzed the population in southern China and lack representation from other regions of the country. Future studies should involve patients from the greater China region. Second, the SNPs of P2Y12R have not been reported in epilepsy. Hence, the discussion concerning the SNPs of P2Y12R is limited. Further validation and studies are necessary to confirm the relationship between P2Y12R and epilepsy. Third, this study was confined to the association of SNPs with epilepsy and lacked specific epileptic subtypes due to the limited sample size. Thus, more research is warranted to improve our understanding of the association between P2Y12R and the pathophysiology of epilepsy.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hauser WA (2019) An unparalleled assessment of the global burden of epilepsy. Lancet Neurol. 18(4):322–324. https://doi.org/10.1016/S1474-4422(19)30042-0
Löscher W, Potschka H, Sisodiya SM, Vezzani A (2020) Drug resistance in epilepsy: clinical impact, potential mechanisms, and new innovative treatment options. Pharmacol Rev. 72(3):606–638. https://doi.org/10.1124/pr.120.019539
Ding D, Zhou D, Sander JW, Wang W, Li S, Hong Z (2021) Epilepsy in China: major progress in the past two decades. Lancet Neurol. 20(4):316–326. https://doi.org/10.1016/S1474-4422(21)00023-5
Siewe JNF, Ukaga CN, Nwazor EO et al (2019) Low prevalence of epilepsy and onchocerciasis after more than 20 years of ivermectin treatment in the Imo River Basin in Nigeria. Infect Dis Poverty. 8(1):8. https://doi.org/10.1186/s40249-019-0517-9
Rees MI (2010) The genetics of epilepsy–the past, the present and future. Seizure. 19(10):680–683. https://doi.org/10.1016/j.seizure.2010.10.029
Pitkänen A, Lukasiuk K (2011) Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol. 10(2):173–186. https://doi.org/10.1016/S1474-4422(10)70310-0
Noebels J (2015) Pathway-driven discovery of epilepsy genes. Nat Neurosci. 18(3):344–350. https://doi.org/10.1038/nn.3933
Thijs RD, Surges R, O’Brien TJ, Sander JW (2019) Epilepsy in adults. Lancet. 393(10172):689–701. https://doi.org/10.1016/S0140-6736(18)32596-0
Wagner AK, Miller MA, Scanlon J, Ren D, Kochanek PM, Conley YP (2010) Adenosine A1 receptor gene variants associated with post-traumatic seizures after severe TBI. Epilepsy Res. 90(3):259–272. https://doi.org/10.1016/j.eplepsyres.2010.06.001
Diamond ML, Ritter AC, Jackson EK et al (2015) Genetic variation in the adenosine regulatory cycle is associated with posttraumatic epilepsy development. Epilepsia. 56(8):1198–1206
Fan X, Chen Y, Li W et al (2020) Genetic polymorphism of ADORA2A is associated with the risk of epilepsy and predisposition to neurologic comorbidity in Chinese southern children. Front Neurosci. 14:590605. https://doi.org/10.3389/fnins.2020.590605
Engel T, Alves M, Sheedy C, Henshall DC (2016) ATPergic signalling during seizures and epilepsy. Neuropharmacology. 104:140–153. https://doi.org/10.1016/j.neuropharm.2015.11.001
Jimenez-Mateos EM, Smith J, Nicke A, Engel T (2019) Regulation of P2X7 receptor expression and function in the brain. Brain Res Bull. 151:153–163. https://doi.org/10.1016/j.brainresbull.2018.12.008
Illes P, Rubini P, Ulrich H, Zhao Y, Tang Y (2020) Regulation of microglial functions by purinergic mechanisms in the healthy and diseased CNS. Cells. 9(5):1108. https://doi.org/10.3390/cells9051108
Illes P, Xu GY, Tang Y (2020) Purinergic signaling in the central nervous system in health and disease. Neurosci Bull. 36(11):1239–1241. https://doi.org/10.1007/s12264-020-00602-7
Huang Z, Xie N, Illes P et al (2021) From purines to purinergic signalling: molecular functions and human diseases. Signal Transduct Target Ther. 6(1):162. https://doi.org/10.1038/s41392-021-00553-z
Jacobson KA, Delicado EG, Gachet C et al (2020) Update of P2Y receptor pharmacology: IUPHAR Review 27. Br J Pharmacol. 177(11):2413–2433. https://doi.org/10.1111/bph.15005
Peng J, Liu Y, Umpierre AD et al (2019) Microglial P2Y12 receptor regulates ventral hippocampal CA1 neuronal excitability and innate fear in mice. Mol Brain 12:71. https://doi.org/10.1186/s13041-019-0492-x
Sipe GO, Lowery RL, Tremblay MÈ, Kelly EA, Lamantia CE, Majewska AK (2016) Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nat Commun. 7:10905. https://doi.org/10.1038/ncomms10905
Lou N, Takano T, Pei Y, Xavier AL, Goldman SA, Nedergaard M (2016) Purinergic receptor P2RY12-dependent microglial closure of the injured blood-brain barrier. Proc Natl Acad Sci U S A. 113(4):1074–1079. https://doi.org/10.1073/pnas.1520398113
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 308(5726):1314–1318. https://doi.org/10.1126/science.1110647
Milior G, Morin-Brureau M, Chali F et al (2020) Distinct P2Y receptors mediate extension and retraction of microglial processes in epileptic and peritumoral human tissue. J Neurosci. 40(7):1373–1388. https://doi.org/10.1523/JNEUROSCI.0218-19.2019
Puchałowicz K, Tarnowski M, Baranowska-Bosiacka I, Chlubek D, Dziedziejko V (2014) P2X and P2Y receptors—role in the pathophysiology of the nervous system. Int J Mol Sci. 15(12):23672–23704. https://doi.org/10.3390/ijms151223672
Eyo UB, Peng J, Swiatkowski P, Mukherjee A, Bispo A, Wu LJ (2014) Neuronal hyperactivity recruits microglial processes via neuronal NMDA receptors and microglial P2Y12 receptors after status epilepticus. J Neurosci. 34(32):10528–10540. https://doi.org/10.1523/JNEUROSCI.0416-14.2014
Gao Y, Yu C, Pi S, Mao L, Hu B (2019) The role of P2Y12 receptor in ischemic stroke of atherosclerotic origin. Cell Mol Life Sci. 76(2):341–354. https://doi.org/10.1007/s00018-018-2937-2
Mo M, Eyo UB, Xie M et al (2019) Microglial P2Y12 receptor regulates seizure-induced neurogenesis and immature neuronal projections. J Neurosci. 39(47):9453–9464. https://doi.org/10.1523/JNEUROSCI.0487-19.2019
Beamer E, Kuchukulla M, Boison D, Engel T (2021) ATP and adenosine-two players in the control of seizures and epilepsy development. Prog Neurobiol. 204:102105. https://doi.org/10.1016/j.pneurobio.2021.102105
Eyo UB, Murugan M, Wu LJ (2017) Microglia-neuron communication in epilepsy. Glia. 65(1):5–18. https://doi.org/10.1002/glia.23006
Illes P, Verkhratsky A, Tang Y (2021) Surveilling microglia dampens neuronal activity: operation of a purinergically mediated negative feedback mechanism. Signal Transduct Target Ther. 6(1):160. https://doi.org/10.1038/s41392-021-00586-4
Timur AA, Murugesan G, Zhang L et al (2012) P2RY1 and P2RY12 polymorphisms and on-aspirin platelet reactivity in patients with coronary artery disease. Int J Lab Hematol. 34(5):473–483. https://doi.org/10.1111/j.1751-553X.2012.01420.x
Pina-Cabral LB, Carvalhais V, Mesquita B et al (2018) Myocardial infarction before and after the age of 45: possible role of platelet receptor polymorphisms. Rev Port Cardiol (Engl Ed). 37(9):727–735. https://doi.org/10.1016/j.repc.2018.03.015
Fisher RS, Acevedo C, Arzimanoglou A et al (2014) ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 55(4):475–482. https://doi.org/10.1111/epi.12550
Badimon A, Strasburger HJ, Ayata P et al (2020) Negative feedback control of neuronal activity by microglia. Nature. 586(7829):417–423. https://doi.org/10.1038/s41586-020-2777-8
Hollopeter G, Jantzen HM, Vincent D et al (2001) Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 409(6817):202–207. https://doi.org/10.1038/35051599
Fontana P, Dupont A, Gandrille S et al (2003) Adenosine diphosphate-induced platelet aggregation is associated with P2Y12 gene sequence variations in healthy subjects. Circulation. 108(8):989–995. https://doi.org/10.1161/01.CIR.0000085073.69189.88
Kar R, Meena A, Yadav BK, Yadav R, Kar SS, Saxena R (2013) Clopidogrel resistance in North Indian patients of coronary artery disease and lack of its association with platelet ADP receptors P2Y1 and P2Y12 gene polymorphisms. Platelets. 24(4):297–302. https://doi.org/10.3109/09537104.2012.693992
Yang HH, Chen Y, Gao CY (2016) Associations of P2Y12R gene polymorphisms with susceptibility to coronary heart disease and clinical efficacy of antiplatelet treatment with clopidogrel. Cardiovasc Ther. 34(6):460–467. https://doi.org/10.1111/1755-5922.12223
Nie XY, Li JL, Zhang Y et al (2017) Haplotype of platelet receptor P2RY12 gene is associated with residual clopidogrel on-treatment platelet reactivity. J Zhejiang Univ Sci B. 18(1):37–47. https://doi.org/10.1631/jzus.B1600333
Moore CS, Ase AR, Kinsara A et al (2015) P2Y12 expression and function in alternatively activated human microglia. Neurol Neuroimmunol Neuroinflamm. 2(2):e80. https://doi.org/10.1212/NXI.0000000000000080
Cserép C, Pósfai B, Lénárt N et al (2020) Microglia monitor and protect neuronal function through specialized somatic purinergic junctions. Science. 367(6477):528–537. https://doi.org/10.1126/science.aax6752
Lin SS, Tang Y, Illes P, Verkhratsky A (2021) The safeguarding microglia: central role for P2Y12 receptors. Front Pharmacol. 11:627760. https://doi.org/10.3389/fphar.2020.627760
Dong YT, Tang Y (2020) Purinergic signalling mediates the inhibitory effect of microglia on neuronal activity in the brain. Purinergic Signal. 16(4):477–478. https://doi.org/10.1007/s11302-020-09759-2
Suzuki T, Kohyama K, Moriyama K et al (2020) Extracellular ADP augments microglial inflammasome and NF-κB activation via the P2Y12 receptor. Eur J Immunol. 50(2):205–219. https://doi.org/10.1002/eji.201848013
Hiragi T, Ikegaya Y, Koyama R. Microglia after seizures and in epilepsy. Cells. 2018;7(4):26. Published 2018 Mar 28. doi:https://doi.org/10.3390/cells7040026
Kinoshita S, Koyama R (2021) Pro- and anti-epileptic roles of microglia. Neural Regen Res. 16(7):1369–1371. https://doi.org/10.4103/1673-5374.300976
Dogui M, Jallon P, Tamallah JB et al (2003) EPI Sousse: incidence of newly presenting seizures in children in the Region of Sousse. Tunisia. Epilepsia. 44(11):1441–1444. https://doi.org/10.1046/j.1528-1157.2003.14403.x
Christensen J, Vestergaard M, Pedersen MG, Pedersen CB, Olsen J, Sidenius P (2007) Incidence and prevalence of epilepsy in Denmark. Epilepsy Res. 76(1):60–65. https://doi.org/10.1016/j.eplepsyres.2007.06.012
Hesdorffer DC, Logroscino G, Benn EK, Katri N, Cascino G, Hauser WA (2011) Estimating risk for developing epilepsy: a population-based study in Rochester. Minnesota. Neurology. 76(1):23–27. https://doi.org/10.1212/WNL.0b013e318204a36a
Fiest KM, Sauro KM, Wiebe S et al (2017) Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology. 88(3):296–303. https://doi.org/10.1212/WNL.0000000000003509
Dai YJ, Xu ZH, Feng B et al (2014) Gender difference in acquired seizure susceptibility in adult rats after early complex febrile seizures. Neurosci Bull. 30(6):913–922. https://doi.org/10.1007/s12264-014-1482-8
Wang H, Xu P, Liao D et al (2017) Association between NLPR1, NLPR3, and P2X7R gene polymorphisms with partial seizures. Biomed Res Int. 2017:9547902. https://doi.org/10.1155/2017/9547902
Zhu WY, Jiang P, He X et al (2016) Contribution of NRG1 gene polymorphisms in temporal lobe epilepsy. J Child Neurol. 31(3):271–276. https://doi.org/10.1177/0883073815589757
Gómez Morillas A, Besson VC, Lerouet D. Microglia and neuroinflammation: what place for P2RY12?. Int J Mol Sci. 2021;22(4):1636. Published 2021 Feb 6. doi:https://doi.org/10.3390/ijms22041636
Funding
This work was supported by the National Natural Science Foundation of China (81904312); the Science and Technology Program of Sichuan Province, China (2019YJ0329); the Sichuan Provincial Cadre Health Care Committee (2018—207); and the Scientific Research Fund of Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital (2021LY28).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no competing interests.
Ethical approval
The studies involving human participants were reviewed and approved by the Ethics Committee of the Sichuan Academy of Medical Science and Sichuan Provincial People’s Hospital. All procedures performed in this study were in accordance with the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained for all adult study participants; for children under age 18, both the consent of the parents or guardians and the assent of the child were obtained.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Q., Shi, NR., Lv, P. et al. P2Y12 receptor gene polymorphisms are associated with epilepsy. Purinergic Signalling 19, 155–162 (2023). https://doi.org/10.1007/s11302-022-09848-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-022-09848-4