Abstract
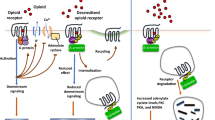
Here, we describe a molecular switch associated with opioid receptors-linked signalling cascades that provides a dual opioid control over P2X3 purinoceptor in sensory neurones. Leu-enkephalin inhibited P2X3-mediated currents with IC50 ~10 nM in ~25 % of small nociceptive rat dorsal root ganglion (DRG) neurones. In contrast, in neurones pretreated with pertussis toxin leu-enkephalin produced stable and significant increase of P2X3 currents. All effects of opioid were abolished by selective μ-opioid receptor antagonist D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP), nonselective inhibitor naloxone, and by PLC inhibitor U73122. Thus, we discovered a dual link between purinoceptors and μ-opioid receptors: the latter exert both inhibitory (pertussis toxin-sensitive) and stimulatory (pertussis toxin-insensitive) actions on P2X3 receptors through phospholipase C (PLC)-dependent pathways. This dual opioid control of P2X3 receptors may provide a molecular explanation for dichotomy of opioid therapy. Pharmacological control of this newly identified facilitation/inhibition switch may open new perspectives for the adequate medical use of opioids, the most powerful pain-killing agents known today.







Similar content being viewed by others
Abbreviations
- AC:
-
adenylate cyclase
- [Ca2+]i :
-
free cytosolic Ca2+ concentration
- cAMP:
-
cyclic adenosine monophosphate
- DRG:
-
dorsal root ganglion
- CTOP:
-
D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2
- DAG:
-
diacylglycerol
- Enk:
-
leu-enkephalin
- GPCRs:
-
G protein-coupled receptors
- InsP3 :
-
inositol 1,4,5-trisphosphate
- meATP:
-
α,β–methylene-ATP
- MORs:
-
μ-opioid receptors
- PKA:
-
protein kinase A
- PKC:
-
protein kinase C
- PLC:
-
phospholipase C
- PTX:
-
pertussis toxin
- TRPV1:
-
transient receptor potential vanilloid 1
References
Wang HB, Zhao B, Zhong YQ, Li KC, Li ZY, Wang Q, Lu YJ, Zhang ZN, He SQ, Zheng HC, Wu SX, Hokfelt TG, Bao L, Zhang X (2010) Coexpression of δ- and μ-opioid receptors in nociceptive sensory neurons. Proc Natl Acad Sci U S A 107:13117–13122
Burnstock G (1996) A unifying purinergic hypothesis for the initiation of pain. Lancet 347:1604–1605
Burnstock G (2013) Purinergic mechanisms and pain—an update. Eur J Pharmacol 716:24–40
Prado FC, Araldi D, Vieira AS, Oliveira-Fusaro MC, Tambeli CH, Parada CA (2013) Neuronal P2X3 receptor activation is essential to the hyperalgesia induced by prostaglandins and sympathomimetic amines released during inflammation. Neuropharmacology 67:252–258
Atkinson TJ, Schatman ME, Fudin J (2014) The damage done by the war on opioids: the pendulum has swung too far. J Pain Res 7:265–268
Sehgal N, Smith HS, Manchikanti L (2011) Peripherally acting opioids and clinical implications for pain control. Pain Physician 14:249–258
Reichert JA, Daughters RS, Rivard R, Simone DA (2001) Peripheral and preemptive opioid antinociception in a mouse visceral pain model. Pain 89:221–227
Shannon HE, Lutz EA (2002) Comparison of the peripheral and central effects of the opioid agonists loperamide and morphine in the formalin test in rats. Neuropharmacology 42:253–261
Stein C, Schafer M, Machelska H (2003) Attacking pain at its source: new perspectives on opioids. Nat Med 9:1003–1008
Hanna MH, Elliott KM, Fung M (2005) Randomized, double-blind study of the analgesic efficacy of morphine-6-glucuronide versus morphine sulfate for postoperative pain in major surgery. Anesthesiology 102:815–821
Dhawan BN, Cesselin F, Raghubir R, Reisine T, Bradley PB, Portoghese PS, Hamon M (1996) International Union of Pharmacology. XII. Classification of opioid receptors. Pharmacol Rev 48:567–592
Murthy KS, Makhlouf GM (1996) Opioid mu, delta, and kappa receptor-induced activation of phospholipase C-β 3 and inhibition of adenylyl cyclase is mediated by Gi2 and Go in smooth muscle. Mol Pharmacol 50:870–877
Endres-Becker J, Heppenstall PA, Mousa SA, Labuz D, Oksche A, Schafer M, Stein C, Zollner C (2007) μ-opioid receptor activation modulates transient receptor potential vanilloid 1 (TRPV1) currents in sensory neurons in a model of inflammatory pain. Mol Pharmacol 71:12–18
Vetter I, Wyse BD, Monteith GR, Roberts-Thomson SJ, Cabot PJ (2006) The μ opioid agonist morphine modulates potentiation of capsaicin-evoked TRPV1 responses through a cyclic AMP-dependent protein kinase A pathway. Mol Pain 2:22
Marker CL, Lujan R, Loh HH, Wickman K (2005) Spinal G-protein-gated potassium channels contribute in a dose-dependent manner to the analgesic effect of μ- and δ- but not κ-opioids. J Neurosci 25:3551–3559
Ocana M, Cendan CM, Cobos EJ, Entrena JM, Baeyens JM (2004) Potassium channels and pain: present realities and future opportunities. Eur J Pharmacol 500:203–219
Schroeder JE, McCleskey EW (1993) Inhibition of Ca2+ currents by a μ-opioid in a defined subset of rat sensory neurons. J Neurosci 13:867–873
Wu ZZ, Chen SR, Pan HL (2004) Differential sensitivity of N- and P/Q-type Ca2+ channel currents to a mu opioid in isolectin B4-positive and -negative dorsal root ganglion neurons. J Pharmacol Exp Ther 311:939–947
McGaraughty S, Honore P, Wismer CT, Mikusa J, Zhu CZ, McDonald HA, Bianchi B, Faltynek CR, Jarvis MF (2005) Endogenous opioid mechanisms partially mediate P2X3/P2X2/3-related antinociception in rat models of inflammatory and chemogenic pain but not neuropathic pain. Br J Pharmacol 146:180–188
Stein C (2013) Opioids, sensory systems and chronic pain. Eur J Pharmacol 716(1-3):179–187
Stein C, Lang LJ (2009) Peripheral mechanisms of opioid analgesia. Curr Opin Pharmacol 9:3–8
Angst MS, Clark JD (2006) Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 104:570–587
DuPen A, Shen D, Ersek M (2007) Mechanisms of opioid-induced tolerance and hyperalgesia. Pain Manag Nurs 8:113–121
Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L (2011) A comprehensive review of opioid-induced hyperalgesia. Pain Physician 14:145–161
Chakrabarti S, Gintzler AR (2007) Phosphorylation of Galphas influences its association with the micro-opioid receptor and is modulated by long-term morphine exposure. Mol Pharmacol 72:753–760
Chakrabarti S, Regec A, Gintzler AR (2005) Biochemical demonstration of mu-opioid receptor association with Gsalpha: enhancement following morphine exposure. Brain Res Mol Brain Res 135:217–224
Pankratov Y, Lalo UV, Dashkin AN, Krishtal A (2001) Heterogeneity of the functional expression of P2X3 and P2X2/3 receptors in the primary nociceptive neurons of rat. Neurochem Res 26:993–1000
Shapovalov G, Gkika D, Devilliers M, Kondratskyi A, Gordienko D, Busserolles J, Bokhobza A, Eschalier A, Skryma R, Prevarskaya N (2013) Opiates modulate thermosensation by internalizing cold receptor TRPM8. Cell Rep 4:504–515
North RA (2004) P2X3 receptors and peripheral pain mechanisms. J Physiol Lond 554:301–308
Pratt EB, Brink TS, Bergson P, Voigt MM, Cook SP (2005) Use-dependent inhibition of P2X3 receptors by nanomolar agonist. J Neurosci 25:7359–7365
Khmyz V, Maximyuk O, Teslenko V, Verkhratsky A, Krishtal O (2008) P2X3 receptor gating near normal body temperature. Pflugers Arch 456:339–347
Grishin EV, Savchenko GA, Vassilevski AA, Korolkova YV, Boychuk YA, Viatchenko-Karpinski VY, Nadezhdin KD, Arseniev AS, Pluzhnikov KA, Kulyk VB, Voitenko NV, Krishtal OO (2010) Novel peptide from spider venom inhibits P2X3 receptors and inflammatory pain. Ann Neurol 67:680–683
Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T (1994) Pharmacological characterization of the cloned κ-, δ-, and μ-opioid receptors. Mol Pharmacol 45:330–334
Chizhmakov I, Yudin Y, Mamenko N, Prudnikov I, Tamarova Z, Krishtal O (2005) Opioids inhibit purinergic nociceptors in the sensory neurons and fibres of rat via a G protein-dependent mechanism. Neuropharmacology 48:639–647
Sanchez-Blazquez P, Gomez-Serranillos P, Garzon J (2001) Agonists determine the pattern of G-protein activation in μ-opioid receptor-mediated supraspinal analgesia. Brain Res Bull 54:229–235
Szucs M, Boda K, Gintzler AR (2004) Dual effects of DAMGO [D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin and CTAP (D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2) on adenylyl cyclase activity: implications for μ-opioid receptor Gs coupling. J Pharmacol Exp Ther 310:256–262
Mo G, Bernier LP, Zhao Q, Chabot-Dore AJ, Ase AR, Logothetis D, Cao CQ, Seguela P (2009) Subtype-specific regulation of P2X3 and P2X2/3 receptors by phosphoinositides in peripheral nociceptors. Mol Pain 5:47
Mo G, Peleshok JC, Cao CQ, Ribeiro-da-Silva A, Seguela P (2013) Control of P2X3 channel function by metabotropic P2Y2 utp receptors in primary sensory neurons. Mol Pharmacol 83:640–647
Park CK, Bae JH, Kim HY, Jo HJ, Kim YH, Jung SJ, Kim JS, Oh SB (2010) Substance P sensitizes P2X3 in nociceptive trigeminal neurons. J Dent Res 89:1154–1159
Paukert M, Osteroth R, Geisler HS, Brandle U, Glowatzki E, Ruppersberg JP, Grunder S (2001) Inflammatory mediators potentiate ATP-gated channels through the P2X3 subunit. J Biol Chem 276:21077–21082
Brown DA, Yule DI (2007) Protein kinase C regulation of P2X3 receptors is unlikely to involve direct receptor phosphorylation. Biochim Biophys Acta 1773:166–175
Delmas P, Crest M, Brown DA (2004) Functional organization of PLC signaling microdomains in neurons. Trends Neurosci 27:41–47
Fujita A, Cheng J, Tauchi-Sato K, Takenawa T, Fujimoto T (2009) A distinct pool of phosphatidylinositol 4,5-bisphosphate in caveolae revealed by a nanoscale labeling technique. Proc Natl Acad Sci U S A 106:9256–9261
Newton PM, Kim JA, McGeehan AJ, Paredes JP, Chu K, Wallace MJ, Roberts AJ, Hodge CW, Messing RO (2007) Increased response to morphine in mice lacking protein kinase C epsilon. Genes Brain Behav 6:329–338
Galeotti N, Stefano GB, Guarna M, Bianchi E, Ghelardini C (2006) Signaling pathway of morphine induced acute thermal hyperalgesia in mice. Pain 123:294–305
Birdsong WT, Arttamangkul S, Clark MJ, Cheng K, Rice KC, Traynor JR, Williams JT (2013) Increased agonist affinity at the μ-opioid receptor induced by prolonged agonist exposure. J Neurosci 33:4118–4127
Acknowledgments
This work was supported by Ukrainian Fund for Fundamental Studies (grant DFFD F46.2/001) and by NIH (grant NIH 1R03TW008228-01A1).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chizhmakov, I., Kulyk, V., Khasabova, I. et al. Molecular mechanism for opioid dichotomy: bidirectional effect of μ-opioid receptors on P2X3 receptor currents in rat sensory neurones. Purinergic Signalling 11, 171–181 (2015). https://doi.org/10.1007/s11302-015-9443-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-015-9443-x