Abstract
Reactive blue 2 (RB-2) had been characterized as a relatively potent ectonucleoside triphosphate diphosphohydrolase (E-NTPDase) inhibitor with some selectivity for NTPDase3. In search for the pharmacophore and to analyze structure-activity relationships we synthesized a series of truncated derivatives and analogs of RB-2, including 1-amino-2-sulfo-4-ar(alk)ylaminoanthraquinones, 1-amino-2-methyl-4-arylaminoanthraquinones, 1-amino-4-bromoanthraquinone 2-sulfonic acid esters and sulfonamides, and bis-(1-amino-4-bromoanthraquinone) sulfonamides, and investigated them in preparations of rat NTPDase1, 2, and 3 using a capillary electrophoresis assay. Several 1-amino-2-sulfo-4-ar(alk)ylaminoanthraquinone derivatives inhibited E-NTPDases in a concentration-dependent manner. The 2-sulfonate group was found to be required for inhibitory activity, since 2-methyl-substituted derivatives were inactive. 1-Amino-2-sulfo-4-p-chloroanilinoanthraquinone (18) was identified as a nonselective competitive blocker of NTPDases1, 2, and 3 (Ki 16–18 μM), while 1-amino-2-sulfo-4-(2-naphthylamino)anthraquinone (21) was a potent inhibitor with preference for NTPDase1 (Ki 0.328 μM) and NTPDase3 (Ki 2.22 μM). Its isomer, 1-amino-2-sulfo-4-(1-naphthylamino)anthraquinone (20), was a potent and selective inhibitor of rat NTPDase3 (Ki 1.5 μM).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extracellular nucleotides such as ATP, adenosine diphosphate (ADP), uridine triphosphate (UTP), and uridine diphosphate (UDP) act as extracellular signaling molecules in virtually all tissues and organs [1]. Especially ATP is involved in a variety of physiological and pathological functions in the body, e.g., as a neurotransmitter and neuromodulator in the central and peripheral nervous system, as a key messenger in nociception, as a tumor-inhibiting agent, in the control of secretion from a variety of endocrine glands, in the modulation of platelet aggregation by ADP, in chloride transport in the airway epithelia, in renal function, in bone and cartilage disease, and as a proinflammatory agent in the immune system [2, 3]. Extracellular nucleotides are efficiently inactivated by surface-located enzymes which sequentially hydrolyze the phosphate groups eventually resulting in the formation of the respective nucleoside and inorganic phosphate [4]. Nucleoside 5′-triphosphates and 5′-diphosphates may be hydrolyzed by members of the E-NTPDase (ectonucleoside triphosphate diphosphohydrolase) family, the E-NPP (ectonucleotide pyrophosphatase/phosphodiesterase) family, and by alkaline phosphatases. Nucleoside 5′-monophosphates are subject to hydrolysis by ecto-5′-nucleotidase and also by alkaline phosphatases [5]. Members of the various ectonucleotidase families reveal overlapping substrate specificity and tissue distribution whose functional significance needs to be further elucidated [4].
E-NTPDases comprise eight members, four of which have two plasma membrane-spanning domains with an active site facing the extracellular milieu [4–6], namely, NTPDase1 (CD39), NTPDase2 (CD39L1), NTPDase3 (CD39L3), and NTPDase8 [7, 8]. In contrast, NTPDase4, 5, 6, and 7 are intracellular membranes proteins. Even though NTPDases5 and 6 may be present at the surface of the plasma membrane and secreted as soluble enzymes following a proteolytic cleavage, their high Km values and low specific activities [9] make it unlikely that these enzymes regulate P2 receptor signaling. NTPDases share five highly conserved sequence domains (apyrase conserved regions) that are presumably of major relevance for their catalytic activity [10]. They contain the actin-HSP 70-hexokinase β- and γ-phosphate binding motif [11, 12].
Members of the E-NTPDase family can hydrolyze nucleoside 5′-triphosphates and nucleoside 5′-diphosphates albeit with varying preference for the individual type of nucleotide [10, 11]. NTPDase1 (CD39, ectoapyrase, ecto-ATP diphosphohydrolase) hydrolyzes ATP and ADP at a molecular ratio of about 1:0.5 to 1:0.9 [13]. In contrast, NTPDase2 has a strong preference for ATP with molecular ratios of ATP:ADP of 1:0.03 or less. NTPDase3 is a functional intermediate and reveals a molecular ratio of ATP:ADP of approximately 1:0.3. The activity of all three types of ectonucleotidases depends on millimolar concentrations of divalent cations such as Ca2+ or Mg2+ [11, 13, 14]. The enzymes hydrolyze not only ATP or ADP but have broad substrate specificity towards purine and pyrimidine nucleotides.
The analysis of nucleotide release requires the availability of inhibitors of ectonucleotidases that should ideally have no effect on P2 receptor activation [6]. It has been demonstrated that stable analogs of ATP can elicit tissue contractions up to 100 times more effectively than ATP. This suggests that the effects of exogenously applied ATP on P2 receptors are limited by its enzymatic degradation. Inhibitors of ectonucleotidases could thus serve as drugs that increase the lifetime of extracellular ATP (or ADP, UTP, or UDP) in situ. They could act in a site- and event-specific manner since they would only have an effect when nucleotides are present.
NTPDase1 is the major physiological E-NTPDase member showing a nearly ubiquitous tissue distribution. It is constitutively expressed in microvascular endothelium and certain immune cells and plays an important role in thromboregulation and immune function [15]. The distribution of NTPDase2 appears to be more restricted. NTPDase2 has been detected on blood vessels [16], cultured brain astrocytes [17], and on certain neuronal progenitor [18] and cancer cells [19, 20]. In contrast, NTPDase3 is expressed in the brain exclusively in neurons, with a predominantly axonal localization. Its colocalization with hypocretin-1/orexin-A indicates that it may be involved in the modulation of feeding, the sleep-wake cycle, or other behavioral components [21]. NTPDase3 has also been reported to be involved in auditory neurotransmission since it is highly expressed in the cochlea [22].
Potent and selective inhibitors for the individual NTPDase isoenzymes are required as pharmacological tools to investigate the (patho)physiological roles of NTPDases. Furthermore, such compounds are required to study their potential as novel drugs, e.g., for the treatment of cancer, as immunomodulatory agents, and for the treatment of cardiovascular or central nervous system disorders. So far only few classes of NTPDase inhibitors have been described [23] (Fig. 1).
These include nucleotide derivatives and analogs, mainly derived from ATP. Until recently, the only compound that has been shown to effectively inhibit the hydrolysis of ATP in a variety of tissues (albeit with moderate potency) without significantly acting on P2 receptors was the structural analog of ATP, ARL 67156 (1, FPL 67156) (N 6-diethyl-β,γ-dibromomethylene-ATP) [24–26]. However, ARL 67156 was recently found to be nearly inactive at rat, mouse, and human NTPDase2 [27, 28]. Although 1 is stable versus ecto-alkaline phosphatases and E-NTPDases, it might be hydrolyzed by E-NPPs, which directly attack the oxygen bridge between the α- and β-phosphorus atom of ATP and related compounds. However, Sévigny and colleagues showed that human NPP1 and NPP3 were unable to hydrolyze ARL 67156 [28]. 8-Thioether-ATP derivatives, found inactive at P2Y and P2X receptors, and stable to NTPDase hydrolysis, have also been described as NTPDase inhibitors. 8-Butylthioadenosine-5′-triphosphate (compound 2) acts as a competitive inhibitor of ATP with an estimated Ki value of 10 μM at NTPDase from bovine spleen [29]. Although the compound appears to be stable against NTPDases, it is likely to be hydrolyzed by the ubiquitously present ectoalkaline phosphatases which accept a very broad variety of substrates. Very recently, we have developed metabolically stable, uncharged nucleotide mimetics derived from uridine 5′-carboxylate and identified PSB-6426 (3) as a selective inhibitor of human NTPDase2 [30].
Besides inhibitors derived from nucleotides, non-nucleotide NTPDase inhibitors have been described. These include suramin (4) and related compounds [13, 31–33], sulfonate dyes such as reactive blue 2 (RB-2, 5) [34–37], and PPADS (6, pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid) [13, 38, 39], which can all attenuate the hydrolysis of ATP. However, all of these compounds are nonselective, as they are also potent antagonists at P2 receptors. We recently identified a novel class of NTPDase inhibitors, the polyoxometalates (POMs), which are inorganic anionic metal complexes, e.g., POM-4 (7) [40]. These compounds have turned out to be useful pharmacological tools [41–43]. However, they have high molecular weights, bear several negative charges, and are not drug-like molecules.
The present study was aimed at identifying the pharmacophore of RB-2 for inhibiting NTPDases and at investigating the structure-activity relationships of truncated RB-2 derivatives. Since RB-2 shows some selectivity for rat NTPDase3 (Ki 1.10 μM, approximately 20-fold selectivity vs NTPDase1 and 2) [27, 44], we expected that this strategy might lead to the identification of an NTPDase3-selective inhibitor.
Results and discussion
Syntheses
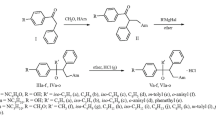
The 1-amino-4-anilino-2-sulfoanthraquinone derivatives 11–19 were synthesized as recently described by combinatorial parallel synthesis using the Ullmann coupling reaction [45]. The new compounds 20–22 were obtained by two different methods. The 1-naphthyl (20) and the 3,4-dimethoxyphenyl derivative (22) were synthesized by the classic Ullmann reaction from bromaminic acid (8) and 1-naphthylamine, or 3,4-dimethoxyphenethylamine, respectively, in water in the presence of potassium carbonate and copper sulfate at 100–105°C (see Fig. 2). The 2-naphthyl derivative (21) was obtained by a new microwave-catalyzed Ullmann reaction in phosphate buffer using elemental copper as a catalyst [46] within only 5 min reaction time (Fig. 2). The 1-amino-2-methyl-4-arylaminoanthraquinone derivatives 23–26 were obtained by solvent-free reaction of 1-amino-4-bromo-2-methylanthraquinone (9) with the appropriate arylamine derivative in the presence of copper acetate and potassium acetate at 110°C for 5–24 h according to a procedure described by Harris et al. [47] (see Fig. 2).
Synthesis of aryl- and arylalkylaminoanthraquinone derivatives 20–22 and 23–26 (for R see Table 1)
Anthraquinone sulfonic acid esters 27–30 and sulfonamides (31–33) were obtained by chlorination of bromaminic acid (8) with phosphorus pentachloride in phosphorus oxychloride to yield the sulfonic acid chloride 10 as a reactive intermediate, which was subsequently treated with the appropriate alcohol or phenol, or ar(alkyl)amine under basic conditions (Fig. 3). When p-ethoxy- or o-methoxy-substituted aniline was used for the condensation with 10, the desired simple sulfonamide derivatives were not obtained. Mass spectral and nuclear magnetic resonance (NMR) analysis revealed that bissulfonamides 34 and 35 had formed instead, although the anilines had been present in the reaction mixtures in excess. The explanation may be an increased nucleophilicity of the aniline nitrogen atom due to the positive mesomeric effects of the substituents (o-methoxy, p-ethoxy).
Solubility and stability of selected anthraquinone derivatives
It was observed that some anthraquinone sulfonic acid sodium salts, which were initially well soluble in water, formed precipitates during the testing. One explanation may be that the high salt concentrations in the buffer solutions, e.g., the presence of calcium ions, which may form hardly soluble calcium sulfonates, reduced their solubility. However, precipitation might also be due to another chemical reaction. Therefore, selected 1-amino-4-phenylamino-2-sulfoanthraquinone derivatives (12, 15, 17, 18) bearing different substituents on the phenyl ring (o-methyl, o, p-dimethyl, o-ethoxy, and p-chloro residues) were investigated for their stability under the test conditions. Aqueous solutions (10 mM) were prepared and diluted with the N-[2-hydroxyethyl]piperazine-N′-2-ethanesulfonic acid (HEPES) buffer that was used in the assays, to obtain a compound concentration of 3 mM. After a short period of time after dilution, compound 18 precipitated, while the other compounds stayed in solution. After 15 h at room temperature, compound 17 also started to precipitate, while 12 precipitated after 48 h and 15 after about 1 week. The precipitated compounds were collected by filtration, dried, and NMR spectra were recorded showing that the precipitated compounds were structurally identical to the original anthraquinone derivatives. This result indicates that the compounds were not degraded, but precipitated upon dilution with buffer solutions due to the lower solubility in the presence of high salt concentrations probably due to the formation of poorly soluble salts of the anthraquinone sulfonic acids with cations in the buffer (HEPES and/or calcium). Since precipitation in most cases only occurred after several hours, testing of high concentrations was always performed with freshly prepared solutions.
Investigation of anthraquinone derivatives as potential inhibitors of nucleoside triphosphate diphosphohydrolases by capillary electrophoresis
Inhibition of rat NTPDases1, 2, and 3 was performed essentially as previously described using a capillary electrophoresis (CE) method [27]. Membrane preparations derived from transfected cells containing rat NTPDase1, NTPDase2, or NTPDase3 were employed. A fixed substrate concentration of 400 μM was used. Test compounds were initially screened at a concentration of 1 mM. For compounds which showed about 50% inhibition or more, IC50 values were determined with six to eight different concentrations of inhibitor spanning about three orders of magnitude. After stopping the reaction by heating, an aliquot was subjected to CE analysis with UV detection at 210 nm. Under the applied conditions less than 10% of substrate was converted by the enzymes. Ki values were calculated from IC50 values as previously described assuming a competitive inhibition mechanism [27].
Structure-activity relationships
Even though the investigated 1-amino-2-sulfo-4-ar(alk)-ylaminoanthraquinone derivatives exhibited only a partial structure of RB-2 and were much smaller than the parent compound, many of them were similarly or even more active as NTPDase inhibitors (Table 1). For example, compound 18 bearing a p-chlorophenylamino residue was a potent, nonselective NTPDase inhibitor (Ki 16–18 μM). While NTPDase2 and 3 were quite tolerant with regard to the substituent in the 4-position accepting a large variety of differentially substituted arylamino residues and even a 3,4-dimethoxyphenethylamino residue, structure-activity relationships (SARs) of NTPDase1 were more restricted: phenylamino (11), m-methylphenylamino (13), and particularly o,p-dimethylphenylamino (15) and p-chlorophenylamino (18) were best tolerated, while monosubstitution in the ortho-position of the phenylamino residue (12, 16, 17) or replacement of the phenyl ring by 1-naphthyl (20) or a 3,4-dimethoxyphenethyl residue (22) were not well tolerated by rat NTPDase1. On the other hand, a 2-naphthyl ring was very well tolerated by NTPDase1: compound 21 was the most potent NTPDase1 inhibitor of the present series with a Ki value of 0.328 μM; it was less potent at NTPDase3 (Ki 2.22 μM) and much less potent at NTPDase2 (19.1 μM). Small p-substituents on the phenyl ring (p-chloro, p-methyl) were well accepted by NTPDase1, while an acetylamino residue in the p-position was detrimental. Compound 20 was the most potent and selective inhibitor of rat NTPDase3 and not inhibitory to rat NTPDase1 and 2 even at high, millimolar concentrations. Rat NTPDase3 appears to have a lipophilic pocket which can accommodate a 1-naphthylamino residue in the 4-position. Concentration-inhibition curves of the most potent nonselective NTPDase inhibitor 18 (PSB-069) at the three investigated NTPDases and of the NTPDase3-selective inhibitor 20 (PSB-06126) are shown in Fig. 4.

An unsubstituted sulfonate group in the 2-position of the anthraquinone scaffold was essential for inhibitory activity. Replacement of the sulfonate by a methyl group (23–26) abolished activity. Bromaminic acid derivatives, in which the sulfonic acid group was converted to sulfonic acid esters (27–30) or sulfonamides (31–33), were also totally inactive. Thus, a negative charge (a free sulfonate group) appeared to be required for NTPDase inhibition. This is in agreement with previous studies on sulfonate dyes and related compounds, which showed that sulfonate groups in certain positions of the molecules were important for ectonucleotidase inhibitory activity [23, 34].
In order to investigate the inhibition mechanism of this class of NTPDase inhibitors, kinetics of NTPDase3 were exemplarily determined in the absence and in the presence of compound 18 (10, 20, and 30 μM). While the Vmax value was not affected by the inhibitor, the Km value increased with increasing concentration of inhibitor indicating a competitive mechanism of inhibition (for Lineweaver-Burk plot see Fig. 5).
The present study is the first one to systematically investigate the SARs of anthraquinone derivatives derived from RB-2 at defined NTPDase isoenzymes. We could confirm previous findings by Tuluc et al. [37] that anthraquinone derivatives with only one substituted phenyl ring at the 4-amino group, which are much smaller than RB-2, can exhibit ectonucleotidase inhibitory activity. However, compound 11 (also known as acid blue 25) was inactive (at 100 μM) in their study investigating ATP degradation in rat vas deferens tissue [37]. Like RB-2, its truncated derivatives also interact with P2 receptors [37, 45, 48]. However, the SARs are clearly different. For example, 16 (PSB-716) was shown to be a potent P2Y2 antagonist (IC50 ca. 9 μM) [45], but it is only a weak NTPDase1 inhibitor. Compound 19 was also a potent inhibitor at P2Y2 (IC50 6–12 μM) [45], but nearly inactive at all three NTPDases tested.
Conclusions
We have investigated 25 anthraquinone derivatives related to RB-2 for their potency at inhibiting rat NTPDases1, 2, and 3 in order to obtain initial information about their structure-activity relationships. While 11 of the compounds had previously been reported, 14 compounds are new and their syntheses and physicochemical characterization are described for the first time. Certain 1-amino-2-sulfo-4-arylaminoanthraquinones were identified as potent NTPDase inhibitors. While the 4-chlorophenylamino derivative 18 was a nonselective inhibitor (Ki 16–18 μM), the 4-(1-naphthylamino) derivative 20 was very potent and selective for rat NTPDase3 versus rat NTPDase1 and 2. In contrast, its 2-naphthylamino isomer 21 was a very potent NTPDase1 inhibitor (Ki 0.328 μM). For future studies it would be of interest to investigate potential species differences of these and other NTPDase inhibitors. Furthermore, it will be important to investigate the potency of some of these compounds at other ectonucleotidases, including ecto-5′-nucleotidase, nucleotide pyrophosphatases/phosphodiesterases (NPPs), and alkaline phosphatases, and to determine their profile at the various P2Y and P2X receptor subtypes, in order to be able to fully assess their usefulness as pharmacological tools. The compounds described will be good starting points for further optimization.
Abbreviations
- ARL 67156:
-
N 6-diethyl-β,γ-dibromomethylene-ATP
- CE:
-
capillary electrophoresis
- DMSO:
-
dimethyl sulfoxide
- EI:
-
electron impact
- ESI:
-
electrospray ionization
- HEPES:
-
N-[2-hydroxyethyl]piperazine-N′-2-ethanesulfonic acid
- HPLC:
-
high-performance liquid chromatography
- HRMS:
-
high-resolution mass spectrometry
- NMR:
-
nuclear magnetic resonance
- (E)-NTPDase:
-
(ecto)-nucleoside 5′-triphosphate diphosphohydrolase
- PPADS:
-
pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid
- RB-2:
-
reactive blue 2
- SAR(s):
-
structure-activity relationship(s)
References
Burnstock G (2007) Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87:659–797
Zimmermann H (1999) Two novel families of ectonucleotidases: molecular structures, catalytic properties and a search for function. Trends Pharmacol Sci 20:231–236
Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Höpner S, Centonze D, Bernardi G, Dell’Acqua ML, Rossini PM, Battistini L, Rötzsche O, Falk K (2007) Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 110:1225–1235
Zimmermann H (2000) Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol 362:299–309
Zimmermann H (2006) Ectonucleotidases in the nervous system. Novartis Found Symp 276:113–128
Zimmermann H (2001) Ectonucleotidases: some recent developments and a note on nomenclature. Drug Devt Res 52:44–56
Knowles AF, Li C (2006) Molecular cloning and characterization of expressed human ectonucleoside triphosphate diphosphohydrolase 8 (E-NTPDase8) and its soluble extracellular domain. Biochemistry 45:7323–7333
Fausther M, Lecka J, Kukulski F, Lévesque SA, Pelletier J, Zimmermann H, Dranoff JA, Sévigny J (2007) Cloning, purification, and identification of the liver canalicular ecto-ATPase as NTPDase8. Am J Physiol Gastrointest Liver Physiol 292:G785–G795
Mulero JJ, Yeung G, Nelken ST, Ford JE (1999) CD39-L4 is a secreted human apyrase, specific for the hydrolysis of nucleoside diphosphates. J Biol Chem 274:20064–20067
Kukulski F, Komoszynski M (2003) Purification and characterization of NTPDase1 (ecto-apyrase) and NTPDase2 (ecto-ATPase) from porcine brain cortex synaptosomes. Eur J Biochem 270:3447–3454
Heine P, Braun N, Sevigny J, Robson SC, Servos J, Zimmermann H (2001) The C-terminal cysteine-rich region dictates specific catalytic properties in chimeras of the ectonucleotidases NTPDase1 and NTPDase2. Eur J Biochem 268:364–373
Kirley TL, Crawford PA, Smith TM (2006) The structure of the nucleoside triphosphate diphosphydrolases (NTPDases) as revealed by mutagenic and computational modelin analyses. Purinergic Signalling 2:379–389
Heine P, Braun N, Heilbronn A, Zimmermann H (1999) Functional characterization of rat ecto-ATPase and ecto-ATP diphosphohydrolase after heterologous expression in CHO cells. Eur J Biochem 262:102–107
Failer BU, Aschrafi A, Schmalzing G, Zimmermann H (2003) Determination of native oligomeric state and substrate specificity of rat NTPDase1 and NTPDase2 after heterologous expression in Xenopus oocytes. Eur J Biochem 270:1802–1809
Robson SC, Sévigny J, Zimmermann H (2006) The E-NTPDase family of ectonucleotidases: structure-function relationships and pathophysiological significance. Purinergic Signalling 2:409–430
Sévigny J, Sundberg C, Braun N, Guckelberger O, Csizmadia E, Qawi I, Imai M, Zimmermann H, Robson SC (2002) Differential catalytic properties and vascular topography of murine nucleoside triphosphate diphosphohydrolase 1 (NTPDase1) and NTPDase2 have implications for thromboregulation. Blood 99:801–2809
Wink MR, Braganhol E, Tamajusuku ASK, Lenz G, Zerbini LF, Libermann TA, Sévigny J, Battastini AMO, Robson SC (2006) Nucleoside triphosphate diphosphohydrolase-2 (NTPDase2/CD39L1) is the dominant ectonucleotidase expressed by rat astrocytes. Neuroscience 138:421–432
Langer D, Ikehara Y, Takebayashi H, Hawkes R, Zimmermann H (2007) The ectonucleotidases alkaline phosphatase and nucleoside triphosphate diphosphohydrolase 2 are associated with subsets of progenitor cell populations in the mouse embryonic, postnatal and adult neurogenic zones. Neuroscience 150:863–879
Knowles AF, Chiang W-C (2003) Enzymatic and transcriptional regulation of human ecto-ATPase/E-NTPDase 2. Arch Biochem Biophys 418:217–227
Buffon A, Ribeiro VB, Wink MR, Casali EA, Sarkis JJ (2007) Nucleotide metabolizing ecto-enzymes in Walker 256 tumor cells: molecular identification, kinetic characterization and biochemical properties. Life Sci 80:950–958
Belcher SM, Zsarnovszky A, Crawford PA, Hemani H, Spurling L, Kirley TL (2006) Immunolocalization of ecto-nucleoside triphosphate for modulation of multiple homeostatic systems including feeding and sleep-wake behaviours. Neuroscience 137:1331–1346
Vlajkovic SM, Vinayagamoorthy A, Thorne PR, Robson SC, Wang CJ, Housley GD (2006) Noise-induced up-regulation of NTPDase3 expression in the rat cochlea: implications for auditory transmission and cochlear protection. Brain Res 1104:55–63
Gendron FP, Benrezzak O, Krugh BW, Kong Q, Weisman GA, Beaudoin AR (2002) Purine signaling and potential new therapeutic approach: possible outcomes of NTPDase inhibition. Curr Drug Targets 3:229–245
Kennedy C, Westfall TD, Sneddon P (1996) Modulation of purinergic neurotransmission by ecto-ATPase. Semin Neurosci 8:195–199
Dowd FJ, Li LS, Zeng W (1999) Inhibition of rat parotid ecto-ATPase activity. Arch Oral Biol 44:1055–1062
Drakulich DA, Spellmon C, Hexum TD (2004) Effect of the ecto-ATPase inhibitor, ARL 67156, on the bovine chromaffin cell response to ATP. Eur J Pharmacol 485:137–140
Iqbal J, Vollmayer P, Braun N, Zimmermann H, Müller CE (2005) A capillary electrophoresis method for the characterization of ecto-nucleoside triphosphate diphosphohydrolases (NTPDases) and the analysis of inhibitors by in-capillary enzymatic microreaction. Purinergic Signalling 1:349–358
Lévesque SA, Lavoie EG, Lecka J, Bigonnesse F, Sévigny J (2007) Specificity of the ecto-ATPase inhibitor ARL 67156 on human and mouse ectonucleotidases. Br J Pharmacol 152:141–150
Gendron FP, Halbfinger E, Fischer B, Duval M, D’Orléans-Juste P, Beaudoin AR (2000) Novel inhibitors of nucleoside triphosphate diphosphohydrolases: chemical synthesis and biochemical and pharmacological characterizations. J Med Chem 43:2239–2247
Brunschweiger A, Iqbal J, Umbach F, Scheiff AB, Munkonda MN, Sévigny J, Knowles AF, Müller CE (2008) Selective nucleoside triphosphate diphosphohydrolase-2 (NTPDase2) inhibitors: nucleotide mimetics derived from uridine-5′-carboxamide. J Med Chem, in press
Demenis MA, Furriel RP, Leone FA (2003) Characterization of an ectonucleoside triphosphate diphosphohydrolase 1 activity in alkaline phosphatase-depleted rat osseous membranes: possible functional involvement in the calcification process. Biochem Biophys Acta 1646:216–225
Crack BE, Beukers MW, McKechnie KC, Ijzerman AP, Leff P (1994) Pharmacological analysis of ecto-ATPase inhibition: evidence for combined enzyme inhibition and receptor antagonism in P2X-purinoceptor ligands. Br J Pharmacol 113:1432–1438
Bültmann R, Wittenburg H, Pause B, Kurz G, Nickel P, Starke K (1996) P2-purinoceptor antagonists: III. Blockade of P2-purinoceptor subtypes and ecto-nucleotidases by compounds related to suramin. Naunyn Schmiedebergs Arch Pharmacol 354:498–504
Wittenburg H, Bültmann R, Pause B, Ganter C, Kurz G, Starke K (1996) P2-purinoceptor antagonists: II. Blockade of P2-purinoceptor subtypes and ecto-nucleotidases by compounds related to Evans blue and trypan blue. Naunyn Schmiedebergs Arch Pharmacol 354:491–497
Stout JG, Kirley TL (1995) Inhibition of purified chicken gizzard smooth muscle ecto-ATPase by P2 purinoceptor antagonists. Biochem Mol Biol Int 36:927–934
Bültmann R, Starke K (1995) Reactive red 2: a P2y-selective purinoceptor antagonist and an inhibitor of ecto-nucleotidase. Naunyn Schmiedebergs Arch Pharmacol 352:477–482
Tuluc F, Bültmann R, Glänzel M, Frahm AW, Starke K (1998) P2-receptor antagonists: IV. Blockade of P2-receptor subtypes and ecto-nucleotidases by compounds related to reactive blue 2. Naunyn Schmiedebergs Arch Pharmacol 357:111–120
Hoffmann C, Heine P, Pradel G, Kim YC, Jacobson KA, Zimmermann H (2000) Inhibition of ecto-apyrase and ecto-ATPase by pyridoxal phosphate-related compounds. Drug Dev Res 51:153–158
Chen BC, Lee CM, Lin WW (1996) Inhibition of ecto-ATPase by PPADS, suramin and reactive blue in endothelial cells, C6 glioma cells and RAW 264.7 macrophages. Br J Pharmacol 119:1628–1634
Müller CE, Iqbal J, Baqi Y, Zimmermann H, Röllich A, Stephan H (2006) Polyoxometalates—a new class of potent ecto-nucleoside triphosphate diphosphohydrolase (NTPDase) inhibitors. Bioorg Med Chem Lett 16:5943–5947
Köhler C, Eckle T, Faigle M, Grenz A, Mittelbronn M, Laucher S, Hart ML, Robson SC, Müller CE, Eltzschig HK (2007) CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation 116:1784–1794
Grenz A, Zhang H, Hermes M, Eckle T, Klingel K, Huang DY, Müller CE, Robson SC, Osswald H, Eltzschig HK (2007) Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J 21:2863–2873
Coppi E, Pugliese AM, Stephan H, Müller CE, Pedata F (2007) Role of P2 purinergic receptors in synaptic transmission under normoxic and ischaemic conditions in the CA1 region of rat hippocampal slices. Purinergic Signalling 3:203–219
Munkonda MN, Kauffenstein G, Kukulski F, Lévesque SA, Legendre C, Pelletier J, Lavoie EG, Lecka J, Sévigny J (2007) Inhibition of human and mouse plasma membrane bound NTPDases by P2 receptor antagonists. Biochem Pharmacol 74:1524–1534
Weyler S, Baqi Y, Hillmann P, Kaulich M, Hunder AM, Müller IA, Müller CE (2008) Combinatorial synthesis of anilinoanthraquinone derivatives and evaluation as non-nucleotide-derived P2Y2 receptor antagonists. Bioorg Med Chem Lett 18:223–227
Baqi Y, Müller CE (2007) Rapid and efficient microwave-assisted copper(0)-catalyzed Ullmann coupling reaction. Org Lett 9:1271–1274
Harris RM, Mariott GJ, Smith JC (1936) The effect of alkyl groups on the properties of anthraquinone and fluorescein dyes. J Chem Soc 1838–1842. DOI 10.1039/JR9360001838
Glänzel M, Bültmann R, Starke K, Frahm AW (2003) Members of the acid blue 129 family as potent and selective P2Y-receptor antagonists. Drug Dev Res 59:63–71
Bessubetz MK, Rozina VS (1948) Acidic derivatives of anthraquinone. I. Influence of substituents in the phenylamine radical of anthraquinone derivatives on their properties. Zh Prikl Khim 21:1152–1161
I.G. Farbenindustrie AG (1933) Anthraquinone derivatives. French Patent FR 751236 19330829
Kegel B, Braun N, Heine P, Maliszewski CR, Zimmermann H (1997) An ecto-ATPase and an ecto-ATP diphosphohydrolase are expressed in rat brain. Neuropharmacology 36:1189–1200
Vorhoff T, Zimmermann H, Pelletier J, Sévigny J, Braun N (2005) Cloning and characterization of the ecto-nucleotidase NTPDase3 from rat brain: predicted secondary structure and relation to other members of the E-NTPDase family and actin. Purinergic Signalling 1:259–270
Acknowledgements
Y.B. and J.I. were supported by DAAD (Deutscher Akademischer Austauschdienst) scholarships. C.E.M. and Y.B. and H.Z. are grateful for support by the Deutsche Forschungsgemeinschaft (DFG, GRK804 and Zi 140/17–3, respectively). We thank Christina Ising, student of molecular biomedicine, for testing some of the compounds.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Experimental section
Reagents and chemicals
4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES), ATP, MgCl2·6H2O, and tris(hydroxymethyl)aminomethane (Trizma Base), penicillin and streptomycin were purchased from Sigma-Aldrich (Taufkirchen, Germany). Culture medium was obtained from Invitrogen (Karlsruhe, Germany). Leupeptin, pepstatin A, chymostatin, and antipain were received from Calbiochem (Schwalbach, Germany).
Bromaminic acid (8) and 1-amino-4-bromo-2-methylanthraquinone (9) were obtained from Sigma-Aldrich (Taufkirchen, Germany). Aniline derivatives were a gift from Bayer AG (through Fonds der Chemischen Industrie). Liquid aniline derivatives were purified by distillation before use in case they showed a dark brown color. Solvents were dried according to standard methods when required. All other chemicals were obtained from standard commercial sources or prepared as described below. Anthraquinone derivatives 11–19 were prepared as recently published [45, 46].
Instruments
NMR spectra were recorded on a Bruker Avance 500 spectrometer. Electron impact mass spectra (EI-MS) were obtained on an MS 50 instrument of the company A.E.I., or a MAT 95 XL instrument of Thermoquest. Polar compounds were analyzed by electrospray ionization using the API 2000 LC-MS instrument from Applied Biosystems. Elemental analyses were performed with a VarioEL instrument of Elementar Analysensysteme GmbH. Melting points were determined on a Gallenkamp apparatus, or a B530 or a B-545 apparatus from Büchi. Melting points of sodium sulfonates were usually not determined because they are >300°C. Reactions were monitored by thin-layer chromatography (TLC) using silica gel plates F254 and/or RP silica gel plates F254. For microwave reactions a CEM Focused™ Microwave Synthesis type Discover apparatus was used. A freeze dryer (CHRIST ALPHA 1–4 LSC) was used for lyophilization.
Synthetic procedures and analytical data of anthraquinone derivatives
Sodium 1-amino-4-(1-naphthylamino)-9,10-dioxo-9,10-dihydroanthracene 2-sulfonate (20)
Bromaminic acid sodium salt (0.500 g, 1.25 mmol), Na2CO3 (0.175 g), and CuSO4 (0.030 g) were dissolved in 10 ml of water. Then 2 g (14 mmol) of 1-aminonaphthalene was added and the reaction mixture was stirred for 16 h at 105°C. After completion of the reaction the mixture was cooled for 4 h at 4°C, and the formed precipitate was filtered off. The precipitate was washed with 10 ml of water and subsequently with 200 ml of dichloromethane until the filtrate became colorless. The residue was dried under vacuum. Yield: 0.281 g (49%); mp > 300°C. 1H-NMR [dimethyl sulfoxide (DMSO)-d 6 , 500 MHz, 303 K]: δ (ppm) = 7.49 (br, 1H, NH2); 7.50 (d, 1H, 3 J = 7.3 Hz, naphthalene); 7.58–7.63 (m, 3H, naphthalene); 7.80 (s, 1H, C3H); 7.84–7.90 (m, 3H, C6H, C7H, naphthalene); 8.02, 8.08 (m, 2H, naphthalene); 8.32 (m, 2H, C5H, C8H); 10.12 (br, 1H, NH2); 12.51 (s, 1H, NH). 13C-NMR (DMSO-d 6 , 125 MHz, 303 K): δ (ppm) = 109.23, 111.46 (C4a, C9a); 121.55, 121.97 (C2′, C4′); 122.92 (C3); 125.76, 126.20 (2C), 126.24, 126.91, 127.12, 128.67, 129.00 (C5, C8, C5′, C6′, C7′, C8′, C4a′, C8a′); 132.94, 133.38, 133.78, 134.40, 134.48, 135.21 (C6, C7, C8a, C10a, C1′, C3′); 142.58, 143.15, 144.49 (C1, C2, C4); 181.92, 182.95 (C9, C10). FAB m/z (%) = 466 (100, C25H15N2O5SNa); 444 (46, C25H15N2O5S).
Sodium 1-amino-4-(2-naphthylamino)-9,10-dioxo-9,10-dihydroanthracene 2-sulfonate (21)
The synthesis was performed in analogy to a recently described procedure [46]. To a 5-ml microwave reaction vial equipped with a magnetic stirring bar were added bromaminic acid sodium salt (0.081 g, 0.20 mmol) and 1-aminonaphthalene (80 mg, 0.40 mmol), followed by a buffer solution consisting of 4 ml (0.2 M) aq. Na2HPO4 solution (pH 9.6) and 1 ml (0.12 M) of NaH2PO4 (pH 4.2). A catalytic amount (ca. 0.002–0.003 g) of finely powdered elemental copper was added. The mixture was capped and irradiated in the microwave oven (100 W) for 5 min at 120°C. Then the reaction mixture was cooled to room temperature (RT), and the product was purified using the following procedure. The contents of the vial were filtered to remove the elemental copper. Then water (approximately 200 ml) was added to the filtrate and the aqueous solution was extracted with chloroform (200 ml). The extraction procedure was repeated until the organic layer became colorless (2–3 times). Then the aqueous layer was reduced by rotary evaporation to a volume of ca. 10 ml, which was subsequently submitted to flash column chromatography using RP-18 silica gel and water as an eluent. The polarity of the eluent was then gradually decreased by the addition of methanol in the following steps: 20, 40, 60, 80, and 100%. Fractions containing blue product were collected. The pooled product-containing fractions were evaporated under reduced pressure to remove the methanol, and the remaining water was subsequently removed by lyophilization to yield 21 as blue powder. Yield: 0.046 g (50%); mp > 300°C. 1H-NMR: δ 7.49 (m, 3H, naphthalene); 7.76 (d, 1H, naphthalene); 7.86 (m, 2H, 6-H, 7-H); 7.92 (d, 1H, naphthalene); 8.00 (d, 1H, naphthalene); 8.09 (s, 1H, 3-H); 8.29 (m, 2H, 5-H, 8-H); 10.12 (br, 2H, 1-NH2); 12.16 (s, 1H, NH). 13C-NMR: δ 109.4, 112.0 (C4a, C9a); 119.3, 123.1 (C2′, C4′); 123.3 (C3); 125.3, 126.1, 126.2, 126.9, 127.3, 127.8, 129.6, 130.5 (C5, C8, C5′, C6′, C7′, C8′, C4a′, C8a′); 133.0, 133.4, 133.7, 134.0, 134.3, 137.1 (C6, C7, C8a, C10a, C1′, C3′); 140.7, 142.90, 144.6 (C1, C2, C4); 182.0 (C9), 182.8 (C10). LC-MS (m/z): 462 [M-Na+NH4 +]+, 445 [M-Na]+, 443 [M-Na]-. Purity by high-performance liquid chromatography (HPLC)-UV (254 nm)-electrospray ionization (ESI)-MS: 98%.
Sodium 1-amino-4-(3,4-dimethoxyphenethylamino)-9,10-dioxo-9,10-dihydroanthracene 2-sulfonate (22)
Bromaminic acid sodium salt (1.0 g, 2.5 mmol), Na2CO3 (0.210 g), and CuSO4 (0.070 g) were suspended in 20 ml of water. Then 3,4-dimethoxyphenethylamine (670 μl, 0.725 g, 4.0 mmol) was added and the reaction mixture was heated at 60°C. Then another portion of 3,4-dimethoxyphenethylamine (1 ml, 1.09 g, 6 mmol) was added and the reaction mixture was stirred for 6 h at 100°C. After the reaction was completed the mixture was cooled and neutralized by the addition of diluted aq. HCl solution. Then 20 ml of water containing 1 ml of saturated aq. NaCl solution were added. The blue-colored solid material was filtered off and washed twice with 20 ml of a saturated NaCl solution, and subsequently three times with 20 ml of dichloromethane each. The residue was dissolved in 200 ml of a mixture of hot water:methanol (1:3) and filtered. The filtrate was evaporated to dryness yielding a blue-violet product, which was further purified by preparative HPLC in analogy to a described procedure [45]. Yield: 54%; mp > 300°C. 1H-NMR (DMSO-d 6 , 303 K, 500 MHz): δ (ppm) = 2.90 (t, 2H, 3 J = 6.9 Hz, ArCH2); 3.63 (dt, 3 J HNCH = 5.3 Hz, 3 J CH2CH2 = 6.9 Hz NHCH 2); 3.71 (s, 3H, C3′-OCH3 or C4′-OCH3); 3.77 (s, 3H, C3′-OCH3 or C4′-OCH3); 6.84 (dd, 1H, 3 J = 8.0 Hz, 4 J = 1.7 Hz, C6′H); 6.89 (d, 1H, 3 J = 8.2 Hz, C5′H); 6.97 (d, 1H, 4 J = 1.6 Hz, C2′H); 7.40 (br, 1H, NH2); 7.77–7.80 (m, 2H, C6H, C7H); 7.81 (s, 1H, C3H); 8.19–8.26 (m, 2H, C5H, C8H); 10.08 (br, 1H, NH2); 10.76 (t, 1H, 3 J = 5.2 Hz, NH).13C-NMR (DMSO-d 6 , 303 K, 125 MHz): δ (ppm) = 34.88 (ArCH2); 44.35 (NHCH2); 55.49, 55.68 (C3′-OCH3, C4′-OCH3); 109.07, 109.29 (C4a, C9a); 112.23, 112.94 (C2′, C5′); 120.70, 121.02 (C3, C6′); 125.79, 126.01 (C5, C8); 131.52 (C1′); 132.56, 132.64, 134.05, 134.10 (C6, C7, C8a, C10a); 143.20, 143.72, 145.06 (C1, C2, C4); 147.57, 148.87 (C3′, C4′); 180.97, 181.74 (C9, C10).
General procedure for the synthesis of 4-substituted 1-amino-2-methylanthraquinone derivatives (23–26)
1-Amino-4-bromo-2-methylanthraquinone (9), potassium acetate, and copper(I) acetate were put together into a mortar and pestled until a finely ground powder was obtained. The mixture was then transferred to a round-bottom flask and the appropriate aniline, or aminonaphthalene derivative, respectively, was added. The mixture was then heated at 100°C under an argon atmosphere upon stirring for 5–24 h. After cooling, ethanol (5–10 ml) was added and the solid residue was collected by filtration, washed with ethanol, 2 N aqueous HCl solution, and water (15 ml each) and finally recrystallized from pyridine (if necessary).
1-Amino-2-methyl-4-phenylaminoanthraquinone (23)
The compound was synthesized from 500 mg (1.6 mmol) of 9 and 2 ml (2.04 g, 22 mmol) of aniline in the presence of 350 mg (3.6 mmol) of potassium acetate and 30 mg (0.16 mmol) of copper(I) acetate. Reaction time: 5 h.
Yield: 331 mg (64%); mp: 243°C. 1H-NMR (DMSO-d 6 , 500 MHz) δ (ppm) = 2.25 (d, 3H, 4 J = 0.6 Hz, CH3); 7.18 (tt, 1H, 3 J = 7.4 Hz, 4 J = 1.2 Hz C4′H); 7.31 (dm, 2H, 3 J = 7.6 Hz, C2′H, C6′H); 7.43 (tm, 2H, 3 J = 7.9 Hz, C3′H, C5′H); 7.44 (s, 1H, 4 J = 0.6 Hz, C3H); 7.82 (dd, 1H, 3 J = 6.3 Hz, 4 J = 2.1 Hz, C6H or C7H); 7.83 (dd, 1H, 3 J = 6.3 Hz, 4 J = 2.1 Hz, C6H or C7H); 8.24–8.29 (m, 2H, C5H, C8H), 8.49 (br, 2H, NH2); 12.25 (s, 1H, NH). 13C-NMR (DMSO-d 6 , 125 MHz, 303 K): δ (ppm) = 18.68 (CH3); 108.08, 109.62 (C4a, C9a); 123.19 (2C, C2′, C6′); 124.43 (C4′); 124.52 (C3); 126.00, 126.13 (C5, C8); 129.82 (2C, C3′, C5′); 132.83, 133.03 (C6, C7); 133.96, 134.12 (C8a, C10a); 137.55 (C2); 139.40 (C1′); 142.02 (C4); 147.23 (C1); 181.97, 182.04 (C9, C10). The assignment of the signals was based on one- and two-dimensional NMR spectra; however, some signals could not be unambiguously assigned.
EI-MS: m/z (%) = 328 (100; [C21H16N2O2]+·); 311 (8; [C21H13NO2]+). High-resolution mass spectrometry (HRMS) (70 eV, EI): Calcd.: 328.121178. Found 328.1209 ⇒ C21H16N2O2. Anal. Calcd. for C21H16N2O2∙0.25H2O: C, 75.77; H, 5.00; N, 8.42. Found: C, 75.96; H, 4.86; N, 8.45.
1-Amino-2-methyl-4-(3-methylphenylamino)anthraquinone (24)
The compound was synthesized from 500 mg (1.6 mmol) of 9 and 2 ml (1.96 g, 18.29 mmol) of 3-methylaniline in the presence of 350 mg (3.6 mmol) of potassium acetate and 30 mg (0.16 mmol) of copper(I) acetate. Reaction time: 24 h.
Yield: 384 mg (71%); mp: 255°C. 1H-NMR (DMSO-d 6 , 500 MHz) δ (ppm) = 2.25 (d, 3H, 4 J = 0.6 Hz, 2-CH3); 2.33 (s, 3H, 3′-CH3); 7.00 (d, 1H, 3 J = 7.3 Hz, C4′H or C6′H); 7.11 (d, 1H, 3 J = 7.3 Hz, C4′H or C6′H); 7.11 (s, 1H, C2′H); 7.31 (t, 1H, 3 J = 7.9 Hz, C5′H); 7.43 (d, 1H, 4 J = 0.6 Hz, C3H); 7.80–7.84 (m, 2H, C6H, C7H); 8.25–8.27 (m, 2H, C5H, C8H); 12.24 (s, 1H, NH). 13C-NMR (DMSO-d 6 , 125 MHz, 303 K): δ (ppm) = 18.69 (2-CH3); 21.13 (3′-CH3); 108.05, 109.48 (C4a, C9a); 120.32 (C6′); 123.85 (C2′ or C4′); 124.63 (C3); 125.27 (C2′ or C4′); 126.00, 126.13 (C5, C8); 129.59 (C5′); 132.82, 133.00 (C6, C7); 133.99, 134.12 (C8a, C10a); 137.56 (C2); 139.27, 139.37 (C1′, C3′); 142.22 (C4); 147.21 (C1); 181.86, 182.01 (C9, C10). EI-MS: m/z (%) = 342.2 (100; C22H18N2O2 +·); 325.2 [3; (C22H8N2O2-NH3)+]. HRMS (70 eV, EI): Calcd.: 342.1368. Found 342.1367 ⇒ C22H18N2O2. Anal. Calcd. for C22H18N2O2∙0.75H2O: C, 74.24; H, 5.52; N, 7.87. Found: C, 73.98; H, 5.28; N, 8.16.
1-Amino-2-methyl-4-(4-methylphenylamino)anthraquinone (25) [49]
The compound was synthesized from 350 mg (1.1 mmol) of 9 and 2.6 g (24.3 mmol) of 4-methylaniline in the presence of 245 mg (3.6 mmol) of potassium acetate and 21 mg (0.16 mmol) of copper(I) acetate. Reaction time: 5 h.
Yield: 260 mg (68%). 1H-NMR (DMSO-d 6 , 500 MHz) δ (ppm) = 2.24 (s, 3H, 2-CH3); 2.32 (s, 3H, 4′-CH3); 7.20 (dm, 2H, 3 J = 8.5 Hz, C2′H, C6′H or C3′H, C5′H); 7.24 (dm, 1H, 3 J = 8.2 Hz, C2′H, C6′H or C3′H, C5′H); 7.37 (s, 1H, C3H); 7.80–7.84 (m, 2H, C6H, C7H); 8.25–8.28 (m, 2H, C5H, C8H); 12.26 (s, 1H, NH). 13C-NMR (DMSO-d 6 , 125 MHz, 303 K): δ (ppm) = 18.69 (2-CH3); 20.65 (3′-CH3); 107.98, 109.11 (C4a, C9a); 123.70 (2C, C2′, C6′); 124.38 (C3); 125.97, 126.12 (C5, C8); 130.30 (2C, C3′, C5′); 132.78, 133.91 (C6, C7); 133.99, 134.03, 134.11 (C8a, C10a, C4′); 136.58, 137.64, (C2, C1′); 142.75 (C4); 147.12 (C1); 181.61, 182.94 (C9, C10).
1-Amino-2-methyl-4-(1-naphthylamino)anthraquinone (26)
The compound was synthesized from 400 mg (1.3 mmol) of 9 and 2.5 g (17 mmol) of 1-aminonaphthalene in the presence of 280 mg (2.85 mmol) of potassium acetate and 25 mg (0.14 mmol) of copper(I) acetate. Reaction time: 12 h. The product is recrystallized from pyridine.
Yield: 123 mg (20%). 1H-NMR (DMSO-d 6 , 500 MHz) δ (ppm) = 2.19 (d, 3H, 4 J = 0.6 Hz, 2-CH3); 7.27 (d, 1H, 4 J = 0.6 Hz C3H); 7.55–7.62 (m, 4H, naphthalene); 7.82–7.85 (m, 3H, C6H, C7H, naphthalene); 8.02 (m, 1H, naphthalene); 8.07 (m, 1H, naphthalene); 8.31 (m, 2H, C5H, C8H); 12.74 (s, 1H, NH). 13C-NMR (DMSO-d 6 , 125 MHz): δ (ppm) = 18.63 (2-CH3); 108.00, 109.61 (C4a. C9a); 121.35, 121.95 (C2′, C4′); 124.67 (C3); 125.45, 126.10, 126.19, 126.27, 126.82, 127.00, 128.63, 128.81 (C5, C8, C5′, C6′, C7′, C8′, C4a′, C8a′); 132.84, 133.09, 134.01, 134.22, 134.45, 135.16 (C6, C7, C8a, C10a, C1′, C3′); 137.76 (C2); 143.48 (C4); 147.24 (C1); 181.99, 182.27 (C9, C10). EI-MS: m/z (%) = 378.2 (100; C25H18N2O2 +·). HRMS (70 eV, EI): Calcd.: 378.1368. Found 378.1365 ⇒ C25H18N2O2.
Synthesis of the sulfonic acid chloride of bromaminic acid (10) [50]
Bromaminic acid sodium salt (8, 10 g, ca. 25 mmol) was suspended in 100 ml of phosphorus oxychloride (POCl3) and phosphorus pentachloride (PCl5, 9 g, 43 mmol) was added. The reaction mixture was stirred at 95°C under argon for 6 h. The remaining POCl3 was distilled off under reduced pressure. Two different methods have been used to isolate the product: (1) the residue was cooled in an ice bath and the cold mixture was poured into 200 ml of ice water. The precipitated solid was filtered off and dried under vacuum and stored until use; (2) the residue was taken up in dichloromethane and the resulting solution was washed three times with 40 ml of ice water each. The organic phase was dried over anhydrous magnesium sulfate and after filtration, the solvent was distilled off under vacuum. The residue obtained was dried and used in the next step without further purification. Yield: 90–96% (raw product); mp approximately 190°C (decomposition).
1-Amino-4-bromoanthraquinone-2-sulfonic acid methyl ester (27)
The sulfonic acid chloride 10 (250 mg, 0.6 mmol) was suspended in 50 ml of methanol, and sodium methoxide (approximately 500 mg) was added. The reaction mixture was stirred under an argon atmosphere for 16 h at RT and subsequently evaporated to dryness. The residue was suspended in a mixture of cyclohexane and ethyl acetate (37:3), silica gel (5 g) was added, and the solvent was removed by rotary evaporation. The material absorbed on silica gel was put onto a silica gel column and the product was purified by column chromatography using cyclohexane:ethyl acetate (37:3) as eluent.
Yield: 143 mg (58%); 1H-NMR (DMSO-d 6 , 500 MHz, 303 K): δ (ppm) = 3.97 (s, 3H, OCH3); 7.52 (br, 1H, NH2); 7.83 (td, 1H, 3 J = 7.4 Hz, 4 J = 1.6 Hz, C6H or C7H); 7.87 (td, 1H, 3 J = 7.4 Hz, 4 J = 1.7 Hz, C6H or C7H); 8.04 (s, 1H, C3H); 8.09 (dd, 1H, 3 J = 7.6 Hz, 4 J = 1.3 Hz, C5H or C8H); 8.16 (dd, 1H, 3 J = 7.6 Hz, 4 J = 1.3 Hz, C5H or C8H); 9.56 (br, 1H, NH2). 13C-NMR (DMSO-d 6 , 125 MHz, 303 K): δ (ppm) = 62.24 (OCH3); 106.21, 114.79 (C4a, C9a); 126.17, 126.46 (C5, C8); 131.40 (C4); 133.10, 133.68, 133.76, 134.31 (C6, C7, C8a, C10a); 138.63, 138.93 (C2, C3); 148.4 (C1); 182.16, 183.70 (C9, C10).
General procedure for the synthesis of 1-amino-4-bromoanthraquinone-2-sulfonic acid nitrophenyl esters
The sulfonic acid chloride 10 (285 mg, 0.7 mmol) was suspended in 33 ml of freshly distilled dichloromethane and completely dissolved by stirring it under an argon atmosphere for 30 min. Then a solution of 430 mg (3.1 mmol) of the appropriate nitrophenol and 4 ml of triethylamine in 8 ml of dichloromethane were added (producing white fumes). The reaction mixture was stirred until the starting compound 10 had completely disappeared (TLC control). The solvent was evaporated and the residue was dissolved in 10 ml of a mixture of dichloromethane and cyclohexane (7:2). Subsequent column chromatography on silica gel using the same solvent mixture gave the pure products.
1-Amino-4-bromoanthraquinone-2-sulfonic acid 2-nitrophenyl ester (28)
Yield: 96 mg (27%); mp 213 – 214°C. 1H-NMR (DMSO-d 6 , 303 K, 500 MHz): δ (ppm) = 7.32 (dd, 1H, 3 J = 8.3 Hz, 4 J = 1.2 Hz, C6′H); 7.63 (ddd, 1H, 3 J = 8.2 Hz, 3 J = 7.6 Hz, 4 J = 1.3 Hz, C5′H); 7.74 (ddd, 3 J = 8.2 Hz, 3 J = 7.6 Hz, 4 J = 1.6 Hz, C4′H); 7.89 (s, 1H, C3H); 7.91 (td, 1H, 3 J = 7.3 Hz, 4 J = 1.7 Hz, C6H or C7H); 7.94 (td, 1H, 3 J = 7.4 Hz, 4 J = 1.8 Hz, C6H or C7H); 8.10 (ddm, 1H, 3 J = 7.1 Hz, 4 J = 1.8 Hz, C5H or C8H); 8.14 (dd, 1H, 3 J = 8.0 Hz, 4 J = 1.7 Hz, C3′H); 8.20 (ddm, 1H, 3 J = 7.1 Hz, 4 J = 1.8 Hz, C5H or C8H). 13C-NMR (DMSO-d 6 , 125 MHz, 303 K): δ (ppm) = 104.08 (C9a); 118.13 (C4a); 120.62 (C4); 124.87 (C3′ or C6′); 126.48, 126.58 (C5, C8); 126.60 (C3′ or C6′); 129.44 (C4′); 132.85, 133.62, 134.54, 134.84 (C6, C7, C8a, C10a); 135.65 (C5′); 137.62 (C2); 139.95 (C2′); 142.23 (C3); 142.90 (C1′); 148.21 (C1); 182.15, 184.42 (C9 und C10). EI-MS: m/z (%) = 502.0 + 504.0 (ratio 1:1; 100; [C20H11BrN2O7S]+·); 380.9+382.9 (ratio 1:1; 31; [C14H8BrNO5S]+·); 300.0+301.9 (ratio 1:1; 84; [C14H7BrNO2]+); 221.1 (41; [C14H7NO2]+·). HRMS (70 eV, EI): Calcd.: 501.9470. Found: 501.9471 ⇒ C20H11BrN2O7S. Anal. Calcd. for C20H11BrN2O7S: C, 47.73; H, 2.20; N, 5.57. Found: C, 47.81; H, 2.26; N, 5.52
1-Amino-4-bromoanthraquinone-2-sulfonic acid 3-nitrophenyl ester (29)
Yield: 215 mg (61%); mp 189°C. 1H-NMR (DMSO-d 6 , 303 K, 500 MHz): δ (ppm) = 7.65 (ddd, 1H, 3 J = 8.2 Hz, 4 J = 2.4 Hz, 5 J = 1.1 Hz, C6′H); 7.71 (t, 1H, 3 J = 8.3 Hz, C5′H); 7.89 (td, 1H, 3 J = 7.3 Hz, 4 J = 1.7 Hz, C6H or C7H); 7.92 (td, 1H, 3 J = 7.4 Hz, 4 J = 1.7 Hz, C6H or C7H); 8.01 (s, 1H, C3H); 8.07 (ddm, 1H, 3 J = 7.0 Hz, 4 J = 2.0 Hz, C5H or C8H); 8.10 (t, 4 J = 2.4 Hz, C2′H); 8.16 (ddm, 1H, 3 J = 7.6 Hz, 4 J = 1.6 Hz, C5H or C8H); 8.22 (ddd, 1H, 3 J = 8.2 Hz, 4 J = 2.1 Hz, 5 J = 1.1 Hz, C4′H). 13C-NMR (DMSO-d 6 , 125 MHz, 303 K): δ (ppm) = 104.28 (C9a); 117.78 (C2′); 117.88 (C4a); 120.72 (C4); 123.15 (C4′); 126.44, 126.57 (C5, C8); 128.83 (C6′); 131.94 (C5′); 132.83, 133.21, 134.50, 134.78 (C6, C7, C8a, C10a); 137.44 (C2); 142.54 (C3); 148.11 (C1); 148.32, 148.67 (C1′, C3′); 182.03, 184.40 (C9, C10). EI-MS: m/z (%) = 502.0+504.0 (ratio 1:1; 46; [C20H11BrN2O7S]+·); 424 (84; [C20H11N2O7S]+·); 300.0+301.9 (ratio 1:1; 45; [C14H7BrNO2]+); 286 (49; [C14H8NO4S]+); 238.0 (61; [C14H8NO3]+); 222.0 (100; [C14H7NO2]+·). HRMS (70 eV, EI): Calcd.: 501.9470. Found: 501.9469 ⇒ C20H11BrN2O7S. Anal. Calcd. for C20H11BrN2O7S: C, 47.73; H, 2.20; N, 5.57. Found: C, 50.98; H, 2.29; N, 5.88.
1-Amino-4-bromoanthraquinone-2-sulfonic acid 4-nitrophenyl ester (30)
Yield: 266 mg (76%); mp 196°C. 1H-NMR (DMSO-d 6 , 303 K, 500 MHz): δ (ppm) = 7.49 (dm, 2H, 3 J = 9.2 Hz, C2′H, C6′H); 7.89 (td, 1H, 3 J = 7.4 Hz, 4 J = 1.6 Hz, C6H or C7H); 7.92 (td, 1H, 3 J = 7.4 Hz, 4 J = 1.8 Hz, C6H or C7H); 7.98 (s, 1H, C3H); 8.08 (ddm, 1H, 3 J = 7.4 Hz, 4 J = 1.6 Hz, C5H or C8H); 8.17 (ddm, 1H, 3 J = 7.4 Hz, 4 J = 1.3 Hz, C5H or C8H); 8.26 (dm, 2H, 3 J = 9.2 Hz, C3′H, C5′H). 13C-NMR (DMSO-d 6 , 125 MHz, 303 K): δ (ppm) = 104.27 (C9a); 117.96 (C4a); 120.81 (C4); 123.54 (2C, C2′, C6′); 126.19 (2C, C3′, C5′); 126.45, 126.58 (C5, C8); 132.85, 133.23, 134.51, 134.79 (C6, C7, C8a, C10a); 137.47 (C2); 142.42 (C3); 146.61 (C4′); 148.03 (C1); 152.65 (C1′); 182.06, 184.44 (C9 und C10). EI-MS: m/z (%) = 502.0+504.0 (ratio 1:1; 30; [C20H11BrN2O7S]+·); 424 (84; [C20H11N2O7S]+·); 300.0+301.9 (ratio 1:1; 18; [C14H7BrNO2]+); 286 (52; [C14H8NO4S]+); 238.0 (61; [C14H8NO3]+); 222.0 (100; [C14H7NO2]+·). HRMS (70 eV, EI): Calcd.: 501.9470. Found: 501.9472 ⇒ C20H11BrN2O7S. Anal. Calcd. for C20H11BrN2O7S: C, 47.73; H, 2.20; N, 5.57. Found: C, 47.55; H, 2.20; N, 5.52.
General procedure for the synthesis of bromaminic acid amides (sulfonamides 31–33)
The sulfonic acid chloride 10 was put into a round-bottom flask flushed with argon and freshly dried and distilled dichloromethane or acetone, respectively, was added. The appropriate amine dissolved in a mixture of triethylamine and dichloromethane was added to the suspension resulting in the evolvement of white fumes. The reaction mixture was stirred at RT under an argon atmosphere until starting compound 10 had completely disappeared [TLC monitoring on silica gel using cyclohexane:ethyl acetate (17:3) as an eluent]. Then the solvent was distilled off under reduced pressure and the residue was dissolved in a mixture of dichloromethane:cyclohexane:ethyl acetate (40:51:9 or 10:17:3, respectively) or in dichloromethane:cyclohexane (7:3), respectively, and purified by column chromatography on silica gel using the same solvent mixture.
1-Amino-4-bromoanthraquinone-2-sulfonic acid phenyl amide (31) [49]
The compound was prepared from 0.5 g (1.25 mmol) of 10 in 30 ml of dichloromethane and 1.5 ml (1.53 g, 16.5 mmol) of freshly distilled aniline in 10 ml of dichloromethane. Reaction time: 16 h at RT; purification by column chromatography with dichloromethane:cyclohexane:ethyl acetate (10:17:3) as eluent. Upon evaporation of the product-containing fractions an orange-red colored product precipitated, which was collected by filtration and dried.
Yield: 200 mg (18%); mp 214°C (lit. mp 220°C [48]). 1H-NMR (DMSO-d 6 , 500 MHz, 303 K): δ (ppm) = 7.08 (tt, 3 J = 7.4 Hz, C4′H); 7.11 (m, 2H, C2′H, C6′H); 7.28 (m, 2H, C3′H, C5′H); 7.81 (td, 1H, 3 J = 7.3 Hz, 4 J = 1.7 Hz, C6H or C7H); 7.84 (td, 1H, 3 J = 7.4 Hz, 4 J = 1.7 Hz, C6H or C7H); 8.00 (dd, 1H, 3 J = 7.1 Hz, 4 J = 1.8 Hz, C5H or C8H); 8.08 (s, 1H, C3H); 8.09 (dd, 1H, 3 J = 7.6 Hz, 4 J = 1.6 Hz, C5H or C8H); 10.81 (s, 1H, NH). 13C-NMR (DMSO-d 6 , 125 MHz, 303 K): δ (ppm) = 104.67 (C9a); 116.82 (C4a); 120.68 (2C, C2′, C6′); 125.13 (C4′); 126.26, 126.44 (C5, C8); 126.98 (C4); 129.65 (2C, C3′, C5′); 132.86, 133.30 (C8a, C10a); 134.18, 134.50, 135.33 (C3, C6, C7); 136.51 (C1′); 141.88 (C2); 147.50 (C1); 181.95, 184.28 (C9, C10).
1-Amino-4-bromoanthraquinone-2-sulfonic acid benzylamide (32)
The compound was prepared from 0.5 g (1.25 mmol) of 10 in 20 ml of dichloromethane, and 0.3 ml (0.29 g, 2.75 mmol) of benzylamine and 0.3 ml of triethylamine in 30 ml of dichloromethane. Reaction time: 16 h at RT; purification by column chromatography with dichloromethane:cyclohexane:ethyl acetate (40:51:9) as eluent.
Yield: 215 mg (37%); mp 189°C. 1H-NMR (DMSO-d 6 , 303 K, 500 MHz): δ (ppm) = 4.12 (s, 2H, CH2); 7.02 (t, 3 J = 7.2 Hz, C4′H); 7.14 (t, 2H, 3 J = 7.7 Hz C3′H, C5′H); 7.18 (d, 2H, 3 J = 6.8 Hz C2′H, C6′H); 7.87 (td, 1H, 3 J = 7.2 Hz, 4 J = 1.4 Hz, C6H or C7H); 7.90 (td, 1H, 3 J = 7.4 Hz, 4 J = 1.7 Hz, C6H or C7H); 8.02 (s, 1H, C3H); 8.07 (dd, 1H, 3 J = 7.4 Hz, 4 J = 1.8 Hz, C5H or C8H); 8.14 (dd, 1H, 3 J = 7.5 Hz, 4 J = 1.4 Hz, C5H or C8H); 8.81 (s, 1H, NH). 13C-NMR (DMSO-d 6 , 125 MHz, 303 K): δ (ppm) = 46.06 (CH2); 105.19 (C9a); 116.41 (C4a); 126.30, 126.51 (C5, C8); 127.23 (C4′); 127.72, 128.18 (C2′, C6′, C3′, C5′); 128.96 (C4); 132.83, 133.37 (C8a, C10a); 134.22, 134.62, 134.63 (C3, C6, C7); 136.69 (C1′); 141.51 (C2); 147.39 (C1); 182.07, 184.08 (C9, C10). EI-MS: m/z (%) = 471.9+469.9 (ratio 1:1; 8; [C21H15BrN2O4S]+·); 392.0 (5; [C21H15 N2O4S]+·); 300.9+303 (ratio 1:1; 27; [C14H7BrNO2]+); 223.1 (29; [C14H7NO2]+·); 106.1 (100; [C7H8N]+). HRMS (70 eV, EI): Calcd.: 469.993. Found: 469.992 ⇒ C21H15BrN2O4S. Anal. Calcd. for C21H15BrN2O4S∙1.5 C6H12: C, 60.30; H, 5.57; N, 4.69. Found: C, 60.17; H, 5.21; N, 4.88.
1-Amino-4-bromoanthraquinone-2-sulfonic acid phenethylamide (33)
The compound was prepared from 0.5 g (1.25 mmol) of 10 in 10 ml of dichloromethane, and 0.3 ml (0.29 g, 2.4 mmol) of 2-phenethylamine and 0.3 ml of triethylamine in 30 ml of dichloromethane. Reaction time: 16 h at RT; purification by column chromatography with dichloromethane:cyclohexane:ethyl acetate (40:51:9) as eluent.
Yield: 296 mg (48%); mp 176°C. 1H-NMR (DMSO-d 6 , 303 K, 500 MHz): δ (ppm) = 2.68 (t, 2H, 3 J = 7.1 Hz, ArCH2); 3.15 (t, 2H, 3 J = 7.1 Hz, NHCH2); 7.00 (tt, 3 J = 6.8 Hz, 4 J = 1.9 Hz, C4′H); 7.10–7.16 (m, 4H, C2′H, C3′H, C5′H, C6′H); 7.87 (td, 1H, 3 J = 7.2 Hz, 4 J = 1.4 Hz, C6H or C7H); 7.90 (td, 1H, 3 J = 7.3 Hz, 4 J = 1.7 Hz, C6H or C7H); 8.08 (ddd, 1H, 3 J = 7.3 Hz, 4 J = 1.7 Hz, 5 J = 0.4 Hz, C5H or C8H); 8.09 (s, 1H, C3H); 8.16 (ddd, 1H, 3 J = 7.3 Hz, 4 J = 1.6 Hz, 5 J = 0.3 Hz, C5H or C8H); 8.33 (s, 1H, NH). 13C-NMR (DMSO-d 6 , 125 MHz, 303 K): δ (ppm) = 35.29 (ArCH2); 43.86 (NHCH2); 105.09 (C9a); 116.67 (C4a); 126.23, 126.31, 126.51 (C5, C8, C4′); 128.23 (2C, C2′, C6′ or C3′, C5′); 128.69 (C4); 128.76 (2C, C2′, C6′ or C3′, C5′); 132.90, 133.45 (C8a, C10a); 134.21, 134.60, 134.78 (C3, C6, C7); 138.49 (C1′); 141.24 (C2); 147.45 (C1); 182.15, 184.22 (C9, C10). EI-MS: m/z (%) = 484.0+486.0 (ratio 1:1; 21; [C22H17BrN2O4S]+·); 392.9+394.9 (ratio 1:1; 35; [C15H10BrN2O4S]+); 380+381.9 (ratio 1:1; 68; [C14H8BrN2O4S]+·); 300.0+302.0 (ratio 1:1; 100; [C14H7BrNO2]+); 221.1 (40; [C14H7NO2]+·); 120.2 (27; [C7H8N]+). HRMS (70 eV, EI): Calcd.: 484.00924. Found: 484.0095 ⇒ C22H17BrN2O4S. Anal. Calcd. for C22H17BrN2O4S∙0.5H2O: C, 53.45; H, 3.67; N, 5.67. Found: C, 53.50; H, 3.74; N, 5.56.
Bis-(1-amino-4-bromoanthraquinone-2-sulfonic acid) 4-ethoxyphenylamide (34)
The compound was prepared from 1.0 g (2.5 mmol) of 10 in 80 ml of dichloromethane, and 1.0 ml (1.05 g, 7.7 mmol) of 4-ethoxyaniline and 3 ml of triethylamine in 10 ml of dichloromethane. Reaction time: 16 h at RT; purification by column chromatography with dichloromethane:cyclohexane (7:3) as eluent. Upon concentrating the product-containing fractions under reduced pressure the product precipitated and was filtered off.
Yield: 180 mg (17%); mp 262°C. 1H-NMR (DMSO-d 6 : CDCl3 (approx. 3:1)), 500 MHz, 303 K): δ (ppm) = 1.34 (t, 3H, 3 J = 7.0 Hz, CH3); 4.07 (d, 2H, 3 J = 7.0 Hz, OCH2); 6.93 (dm, 2H, 3 J = 8.9 Hz, C2′H, C6′H or C3′H, C5′H); 7.19 (dm, 2H, 3 J = 8.9 Hz, C2′H, C6′H or C3′H, C5′H); 7.84 (td, 2H, 3 J = 6.4 Hz, 4 J = 2.1 Hz, C6H, C6″H or C7H, C7″H); 7.86 (td, 2H, 3 J = 6.4 Hz, 4 J = 2.1 Hz, C6H, C6″H or C7H, C7″H); 8.01 (s, 2H, C3H, C3″H); 8.04 - 8.08 (m, 2H, C5H, C5″H or C8H, C8″H); 8.09–8.13 (m, 2H, C5H, C5″H or C8H, C8″H); 8.25 (br, 4H, 1-NH2, 1″-NH2). 13C-NMR (DMSO-d 6 : CDCl3 (ca. 3:1)), 125 MHz, 303 K): δ (ppm) = 14.04 (CH3); 63.54 (OCH2); 104.23 (2C, C9a, C9a″); 115.42 (2C, C3′, C5′); 117.63 (2C, C4a, C4a″); 123.15 (C1′); 124.00 (2C, C4, C4″); 126.03, 126.20 (2C each, C5, C5″, C8, C8″); 132.48 (C8a, C8a″ or C10a, C10a″); 132.76 (2C, C2′, C6′); 132.85 (C8a, C8a″ or C10a, C10a″); 133.87, 134.10 (2C each, C6, C6″, C7, C7″); 136.74 (2C, C2, C2″); 143.03 (2C, C3, C3″); 147.82 (2C, C1, C1″); 160.46 (C4′); 181.26, 183.71 (2C each, C9, C9″, C10, C10″). EI-MS: m/z (%) = 867.0+865+863 (ratio 1:2: 1; 11; [C36H23Br2N3O9S]+·); 500+502.0 (ratio 1:1; 34; [C22H17BrN2O5S]+·); 301.0+303 (ratio 1:1; 25; [C14H8BrNO2]+); 136.1 (100; [C8H10NO]+); 108.1 (69; [C6H6NO]+). IR (KBr): ν [cm-1] = 3459, 3420 (NH2); 1669 (C = O); 1587 (Ar); 1499 (Ar); 1390, 1366 (SO2NR2); 1254 (C-O); 1160 (SO2NR2); 889 (Ar); 745, 724 (o-subst. Ar).
Bis-(1-amino-4-bromoanthraquinone-2-sulfonic acid) 2-methoxyphenylamide (35)
The compound was prepared from 1.0 g (2.5 mmol) of 10 in 80 ml of dichloromethane, and 1.0 ml (1.092 g, 8.867 mmol) of 2-methoxyaniline and 3 ml of triethylamine in 10 ml of dichloromethane. Reaction time: 16 h at RT; purification by column chromatography with dichloromethane:cyclohexane (7:3) as eluent.
Yield: 180 mg (14%); mp 271°C. 1H-NMR (DMSO-d 6 , 500 MHz, 303 K): δ (ppm) = 3.60 (s, 3H, CH3); 7.13 (dd, 1H, 3 J = 8.4 Hz, 4 J = 1.3 Hz, C3′H or C6′H); 7.13 (ddd, 1H, 3 J = 8.0 Hz, 3 J = 7.6 Hz, 4 J = 0.7 Hz, C3′H or C4′H); 7.47 (dd, 1H, 3 J = 8.2 Hz, 4 J = 1.6 Hz, C3′H or C6′H); 7.57 (ddd, 1H, 3 J = 8.3 Hz, 3 J = 7.5 Hz, 4 J = 1.7 Hz, C3′H or C4′H); 7.85 (td, 2H, 3 J = 7.2 Hz, 4 J = 1.7 Hz, C6H, C6″H or C7H, C7″H); 7.88 (td, 2H, 3 J = 7.2 Hz, 4 J = 1.8 Hz, C6H, C6″H or C7H, C7″H); 8.01 (ddm, 2H, 3 J = 6.8 Hz, 4 J = 2.1 Hz, C5H, C5″H or C8H, C8″H); 8.08 (ddm, 2H, 3 J = 6.4 Hz, 4 J = 2.5 Hz, C5H, C5″H or C8H, C8″H); 8.08 (s, 2H, C3H, C3″H); 8.35 (br, 4H, 1-NH2, 1″-NH2). 13C-NMR (DMSO-d 6 , 125 MHz, 303 K): δ (ppm) = 55.53 (OCH3); 103.84 (2C, C9a, C9a″); 112.80 (C3′); 117.18 (2C, C4a, C4a″); 119.42, 121.15 (C1′, C5′); 124.15 (2C, C4, C4″); 126.05, 126.20 (2C each, C5, C5″, C8, C8″); 132.45, 132.86, 132.99, 133.19 (C8a, C8a″, C10a, C10a″, C4′, C6′); 134.08, 134.35 (2C each, C6, C6″, C7, C7″); 136.75 (2C, C2, C2″); 143.18 (2C, C3, C3″); 148.13 (2C, C1, C1″); 157.11 (C4′); 181.34, 183.66 (2C each, C9, C9″, C10, C10″). EI-MS: m/z (%) = 848.9+850.9+853 (ratio 1:2: 1; 27; [C35H21Br2N3O9S]+·); 486 + 488.0 (ratio 1:1; 80; [C21H15BrN2O5S]+·); 301.0+303 (ratio 1:1; 34; [C14H8BrNO2]+); 122.1 (100; [C7H8NO]+). IR (KBr): ν [cm-1] = 3424 (NH2); 2845 (OCH3); 1675 (C = O); 1594 (Ar); 1366 (SO2NR2); 1257 (CO); 11.78 (SO2NR2); 891 (Ar); 724 (o-subst. Ar).
NTPDase inhibition assays
Enzyme preparations
The preparation of recombinant rat NTPDase1–3 was performed essentially as described by Iqbal et al. [27]. In brief, Chinese hamster ovary (CHO) cells were transfected by electroporation with plasmid DNA-containing rat NTPDase1 (GenBank Accession number U81295) [51] and NTPDase2 (Y11835) [51] and NTPDase3 (AJ437217) [52], all cloned into the pcDNA3 plasmid. Transfection with the empty plasmid pcDNA3 served as a control. Membrane fractions were prepared in the presence of protease inhibitors from transiently transfected CHO cells 48 h after electroporation. The final pellet fraction was resuspended in 50% (v/v) glycerol containing 2 mM iodoacetamide, 20 mM HEPES (pH 7.4) and stored at -20°C. The membrane preparations contained 4–6 μg of protein/μl.
Enzyme assay
Stock solutions (10 mM or 1 mM) of test compounds were prepared in water or water containing 10% DMSO for the more lipophilic compounds, respectively, and dilutions were made in assay buffer (140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM HEPES, pH 7.4). The reaction mixture for NTPDase inhibition assays contained ATP (final concentration 400 μM) dissolved in assay buffer and test compound (10 μl, different concentrations). The reaction was started by the addition of 10 μl of enzyme-containing membrane preparation to obtain a final volume of 100 μl. Control experiments were performed using membrane preparations of cells transfected with the empty plasmid (pcDNA3). The reaction was carried out at 37°C for 10 min and stopped by heating at 99°C for 3 min. Then 50 μl were transferred to a CE vial and mixed with an UMP solution (final concentration 10 μM, internal standard) in 450 μl of water. The resulting solution was subjected to CE analysis using a P/ACE MDQ CE system (Beckman Instruments, Fullerton, CA, USA) equipped with a UV detection system coupled with a diode array detector. The capillary temperature was kept constant at 25°C. The temperature of the sample storing unit was adjusted to 25°C. The electrophoretic separations were carried out using an eCAP polyacrylamide-coated fused-silica capillary [30 cm (20 cm effective length) × 50 μm internal diameter × 360 μm outer diameter, obtained from CS-Chromatographie (Langerwehe, Germany)]. The separations were performed with phosphate buffer 50 mM, pH 6.5 using an applied current of -60 μA and a data acquisition rate of 8 Hz. Analytes were detected using direct UV absorbance at 210 nm. The capillary was conditioned by rinsing with water for 2 min and subsequently with phosphate buffer for 1 min. Sample injections were made at the cathodic side of the capillary. Data collection and peak area analysis were performed by the P/ACE MDQ software 32 KARAT. Inhibition curves were analyzed by Prism 3.0 (GraphPad, San Diego, CA, USA).
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Baqi, Y., Weyler, S., Iqbal, J. et al. Structure-activity relationships of anthraquinone derivatives derived from bromaminic acid as inhibitors of ectonucleoside triphosphate diphosphohydrolases (E-NTPDases). Purinergic Signalling 5, 91–106 (2009). https://doi.org/10.1007/s11302-008-9103-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-008-9103-5