Abstract
Presynaptic nerve terminals are equipped with a number of presynaptic auto- and heteroreceptors, including ionotropic P2X and metabotropic P2Y receptors. P2 receptors serve as modulation sites of transmitter release by ATP and other nucleotides released by neuronal activity and pathological signals. A wide variety of P2X and P2Y receptors expressed at pre- and postsynaptic sites as well as in glial cells are involved directly or indirectly in the modulation of neurotransmitter release. Nucleotides are released from synaptic and nonsynaptic sites throughout the nervous system and might reach concentrations high enough to activate these receptors. By providing a fine-tuning mechanism these receptors also offer attractive sites for pharmacotherapy in nervous system diseases. Here we review the rapidly emerging data on the modulation of transmitter release by facilitatory and inhibitory P2 receptors and the receptor subtypes involved in these interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ionotropic P2X receptors and metabotropic P2Y receptors are the principal cell surface proteins, conveying the action of extracellular ATP, the ubiquitous signaling substance. P2X receptors are ligand-gated cation channels, composed of three individual subunits, whereas P2Y receptors belong to the superfamily of G protein-coupled receptors (GPCRs), with seven transmembrane domains. Various subtypes of P2X and P2Y receptor families are expressed throughout the brain and involved in a wide array of functions from fast synaptic transmission to long-term plasticity and trophic changes important for development, neuron-glia interactions, and neuroimmunomodulation. In addition, ATP modulates synaptic transmission pre- and postsynaptically, both in a positive and negative direction via activation of P2X and P2Y receptors, respectively.
The presynaptic nerve terminal is an important regulatory site, whereby the efficacy of synaptic transmission could be locally and efficiently controlled. Accordingly, axon terminals in the central nervous system and in the periphery are equipped with a wide variety of auto- and heteroreceptors [1–4]. Whereas presynaptic metabotropic receptors convey negative feedback regulation of transmitter release, presynaptic ionotropic receptors could amplify synaptic transmission. Moreover the activation of ligand-gated cation channels with high Ca2+ permeability could directly elicit transmitter release triggered by the Ca2+ influx through the receptor-ion channel complex [2, 4]. Coactivation of different presynaptic receptors provides a fine-tuning mechanism whereby different neurotransmitters and modulators can mutually influence the activity of each other. Presynaptic and extrasynaptic receptors controlling transmitter release also offer attractive target sites for existing and future pharmacotherapy, as they may modify the normal and pathological synaptic information processing without all-or-none actions [3].
Since ATP and its related nucleotides are ubiquitous signaling molecules, it is not surprising that their receptors, i.e., ionotropic P2X and metabotropic P2Y receptors, participate both in the negative and positive feedback modulation of neurotransmitter release. Although the principal function proposed for ATP-sensitive P2 receptors was that they mediate the fast transmitter action of extracellular ATP in neuro-neuronal and neuro-effector synapses in the nervous system, it was already recognized in the early 1990s that they are also involved in the regulation of transmitter release [5, 6]. It was subsequently revealed that the release of the major neurotransmitters of the brain and the peripheral neurons [acetylcholine (ACh), noradrenaline (NA), dopamine (DA), serotonin, glutamate, γ-aminobutyric acid (GABA)] are modulated by P2X and/or P2Y receptors. In 2000, Cunha and Ribeiro reviewed the literature on the presynaptic modulator role of ATP and suggested that there is a mismatch between the abundance of P2 receptor expression, the robust release of ATP in almost all parts of the central nervous system (CNS) and peripheral nervous system (PNS), and the relative paucity of identified P2 receptor-mediated synapses, which implicates the major role of ATP as a neuromodulator, rather than a classic transmitter [7].
The focus of this mini-review is the facilitatory and inhibitory modulation of neurotransmitter release by different subtypes of P2X and P2Y receptors, irrespective of their localization, i.e., whether they are pre-, post-, or extrasynaptic.
Therefore, in addition to a brief summary of the determining factors of ATP availability in synapses, the structure, pharmacology, signal transduction, and distribution of P2X and P2Y receptors in the nervous system, available information on the release-modulating P2 receptors, and the receptor subtypes involved in these interactions will be detailed and updated.
Determining factors of ATP availability in synapses
The participation of ATP and related nucleotides in the regulation of neurotransmitter release presumes their accumulation in the extracellular space upon ongoing neuronal activity. Extracellular purine availability in the nervous system is basically determined by the balance of release and removal by enzymatic degradation and uptake.
Sources and stimuli that trigger ATP release
Since ATP is ubiquitous, all metabolically active cells of the nervous system provide a potential pool for its release. Therefore, besides the nerve terminals themselves, the cellular source of released purines participating in the modulation of neurotransmitter release could be any cell type located in contact with nerve terminals, i.e., astrocytes, microglia, and endothelia. A wide variety of stimuli are known to release ATP to the extracellular space, which could lead to purine levels sufficiently high to activate nucleotide receptors expressed on the surface of pre- and postsynaptic membranes [8, 9]. Although the stimulation-dependent release of ATP upon conventional [10, 11] and high-frequency (e.g., [12]) neuronal activity is well documented, these stimuli probably result in a spatially restricted, localized increase in extracellular purine levels, which serve the fast synaptic transmission and its modulation within the synaptic cleft. Furthermore, ATP-metabolizing ectoenzymes, present on the nerve terminal membrane, and glial cells [13], such as ectoNTPDases, and the CD39/ecto-5′nucleotidase [14], may strongly limit nucleotide availability under these conditions. On the other hand, pathological events are known also to stimulate purine release. These signals include mechanical [15–17], chemical [18], and hypotonic stimuli [19], hypoxia/hypoglycemia/ischemia and consequent energy deprivation [20–25], inflammatory signals, such as bacterial lipopolysaccharide (LPS) [26, 27], interleukin-1β (IL-1β) [28], and cellular injury. The pathological ATP release might result in a purine-rich extracellular milieu leading to a more widespread activation of receptors reaching also the extrasynaptic receptors on the neighboring nerve terminals or distant cells such as astrocytes. Therefore, P2 receptors could play a role in the modulation of not only neuronal but also astrocytic transmitter release. Finally, nucleotides and nucleosides may promote further release of purines, by a homo- or heteroexchange mechanism, if they reach a relatively high concentration in the extracellular space [29].
Mechanisms of ATP release
Since ATP is a highly polarized molecule, which cannot pass freely the cell membrane being released to the synaptic cleft, it could enter the extracellular space by the following mechanisms: (1) vesicular exocytosis, (2) carrier-mediated release, (3) release through channels and membrane pores, and (4) cytolytic release. (1) Vesicular exocytosis is a prototype mechanism for neurotransmitters and neuromodulators to enter the extracellular space, which is expected to be a [Ca2+]o-dependent process. Indeed, ATP is taken up and stored in synaptic vesicles of nerve terminals [8] and astrocytes [15] and [Ca2+]o-dependent ATP release in response to neuronal stimulation appears in many areas of the central and peripheral nervous system (for further references see [8, 9, 24, 30, 31]). Moreover, recent findings indicate that vesicular ATP could be released not only from nerve terminals but also from neuronal somata [32] and astrocytes [15]. (2) Although specific transporters capable of transmembrane movement of ATP are yet to be molecularly identified in the nervous system, ABC (ATP binding cassette) proteins have been implicated as ATP transporters [19, 33, 34] in non-neuronal cells. These transporters are also expressed in glial cells [35] and mediate ATP release upon hypo-osmotic challenge [36, 37]. (3) Channels and pores, such as connexin hemichannels [38], are also potential candidates to drive the transmembrane movement of ATP. They have been identified to mediate ATP release from astrocytes and other non-neuronal cells in response to mechanical stress [39, 40] and other stimuli [31, 41]. (4) Although only scarcely supported by direct experimental proof [42], the general assumption is that any kind of cellular injury could result in high local ATP concentrations in the extracellular space. In this case the millimolar cytoplasmic ATP is expected to leak out of the cell through the membrane damage.
Metabolism of ATP in the extracellular space
Several enzyme families are responsible for the extracellular degradation of ATP in the nervous system. The first step of the inactivation of ATP is mediated by the family of ectonucleoside triphosphate diphosphohydrolases (ENTPDases, EC 3.6.1.5, also known as ectoATPase or apyrase), which are able to hydrolyze ATP and adenosine diphosphate (ADP) to AMP [14]. Among these enzymes ENTPDase 1, 2, 3, and 8 are present in the brain [43, 44], having low micromolar Km for ATP and ADP giving rise to rapid and highly effective hydrolysis of ATP in almost all neuronal tissues. In addition to the ENTPDase family, ATP and other nucleotides could also be dephosphorylated by ectonucleotide pyrophosphatases (ENPPs) and by alkaline phosphatases, both having broader substrate specificity, but also widespread tissue distribution [14]. The final step of extracellular inactivation is the hydrolysis of AMP by the ecto-5′-nucleotidase (EC 3.1.3.5) enzyme, which is the rate-limiting step giving rise to the formation of adenosine that acts on P1 receptors, which include A1, A2A, A2B, and A3 receptor subtypes. Thus, endogenous ATP is converted to adenosine to activate A1 adenosine receptors within a second in the hippocampus [45, 46], whereas the hydrolysis of ATP seems slower in other brain regions, such as the cerebral cortex [47]. Ectoenzymes therefore have an important role in the substrate delivery to different subtypes of P2X and P2Y receptors. In spite of its short half-life, effective concentrations of nucleotides can be reached in the synapse for the activation of ionotropic P2X receptors and metabotropic P2Y receptors.
Structure, pharmacology, and signal transduction of P2X receptors
Ionotropic P2X receptors are nonselective cation channels consisting of at least three subunits. P2X receptor subunits are 379-595 amino acid long polypeptide chains, having two transmembrane domains (TM1 and TM2) and a large extracellular loop [48, 49]. Until now seven members of this receptor family have been identified molecularly, which are numbered from P2X1 to P2X7, and have individual kinetics and pharmacological phenotype [50]. These receptor proteins coassemble into various homo- or heterooligomeric assemblies to form functional receptors. Among possible combinations so far 16 variations have been proved to be functional [51]. These are all of the homooligomeric receptors, except P2X6, which does not function in homooligomeric form, and the rest are heterooligomers, formed from P2X1-P2X6 subunits. However, recently it has been reported that by N-glycosylation even the homomeric P2X6 receptor could be rendered functional [52]. On the other hand, the P2X7 receptor functions only in homooligomeric form and does not coassemble with other known P2X receptor subunits. P2X receptors are permeable to both monovalent (Na+, K+) and divalent (Ca2+) cations and the activation of the receptor generates an inward current leading to the local depolarization of the cell membrane; in addition, the Ca2+ influx through the receptor-ion channel complex could directly trigger transmitter release. Moreover, upon prolonged or repetitive agonist application certain P2X receptors, especially the P2X7 receptor, display pore dilation which makes the channel permeable to high molecular weight cations up to 800 Da.
Basically P2X receptors are sensitive to ATP and to its various synthetic analogues but not to AMP and adenosine and the ligand binding profiles of homomeric P2X receptors are well established (for further information, see [50, 53]). On the other hand, less is known about the pharmacology of heteromeric receptors; among them, the pharmacological profile of P2X2/3, P2X2/6, P2X1/2, P2X1/4, P2X1/5, and P2X4/6 are described [54–59]. However, the expression pattern and the ligand binding profile of individual assemblies of P2X receptors are highly overlapping, often creating difficulties in the identification of the P2X receptor subunit composition of receptors expressed in native tissues. Moreover P2X receptor assemblies also share common ligand binding properties with certain members of the P2Y receptor family. Therefore, transgenic mice genetically deficient in individual P2X receptor subtypes are being increasingly used for P2X receptor identification.
The distribution of P2X receptors in neuronal structures
In situ hybridization studies with specific riboprobes, and immunocytochemical studies using antibodies raised against individual P2X receptor subunits, revealed that all seven P2X receptors are widely expressed in the nervous system. However, the expression of different receptor subunits show species-, region-, and cell type-specific distinct distribution [60]. Among the P2X receptors, P2X2, P2X4, and P2X6 seem to be most abundantly expressed in the brain, whereas other subunits show more restricted localization [60–63]. The typical localization of the P2X2 receptor is on nerve terminals of the brain and the periphery [61, 64, 65], although it also appears postsynaptically [62]. P2X1 receptors had initially been suggested to be exclusively expressed on smooth muscle membrane consistent with its role in mediating fast synaptic transmission at the autonomic neuroeffector junction [60]. However, recent studies with more sensitive probes revealed that its expression is more widespread, i.e., it is also present on central and peripheral neurons [63, 66]. The same holds true for P2X3 receptors, which are primarily associated with sensory pathways, but functional studies indicate that they are also expressed in other brain regions and autonomic pathways [67–69]. The P2X4 receptor shows substantial expression in several brain areas such as the cerebral cortex, hippocampus, thalamus, and brainstem [70] and is associated with postsynaptic specialization of synaptic contacts [62]. P2X5 subunits have the most restricted localization in the brain, although it shows strong representation in certain areas, e.g., the nucleus tractus solitarii (NTS) [71]. Finally, P2X7 receptors are also expressed in the brain, especially in reactive microglia and astroglia [72] and immunoelectron microscopic studies revealed a widespread presynaptic expression of P2X7 receptor immunoreactivity in a number of different brain areas, including the brainstem, hippocampus, cortex, spinal cord, and the skeletal neuromuscular junction [73–75]. However, two studies, using the same antibodies, demonstrated that P2X7 receptor immunoreactivity is still observable in the brain of P2X7 receptor knockout animals and thereby raised doubts on the validity of previous immunocytochemical observations [76, 77]. Therefore, available P2X7 receptor antibodies either recognize a site, which is not the P2X7 receptor, or, as a recent study indicates [78], a brain analogue of the P2X7 receptor, which shares its antibody binding domain with the cloned P2X7 receptor and partially retains its functionality in P2X7 receptor knockout animals.
Structure, pharmacology, and signal transduction of P2Y receptors
P2Y receptors all belong to G protein-coupled receptors, having seven hydrophobic transmembrane domains, and possess their ATP binding site on the external side of TM3 and TM7 domains [79–82]. The P2Y receptor family has eight individual members, numbered P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14. P2Y receptors are basically activated by adenine and uridine nucleotides, such as ATP, ADP, uridine diphosphate (UDP), and uridine triphosphate (UTP), but not by nucleosides and they are classified according to their sensitivity to purines and/or pyrimidines: P2Y1, 12 and P2Y13 are adenine nucleotide-preferring receptors; P2Y6 is preferred by uridine nucleotides; P2Y2, 4 and P2Y11 are receptors with mixed selectivity; whereas P2Y14 is activated by UDP-glucose, UDP-galactose, UDP-N-acetylglucosamine, and UDP-glucuronic acid [83]. Although a minority of P2Y receptor subtypes are incompletely characterized and the pharmacological profiles of individual P2Y receptors are partially overlapping, ligands are available which display some selectivity to certain subtypes of the P2Y receptor family [for further information see 84, 85]. Nevertheless, identification of individual P2Y receptors requires careful pharmacological analysis and the use of receptor knockout animals, if available, and exclusion of the involvement of adenosine receptors in the effect of nucleotides used as P2Y agonists, because they may be metabolized to adenosine (see, e.g., [45, 47]). In addition, the potential heteromerization of P2Y receptor subtypes with each other and with A1 adenosine receptors [86] should also be taken into account when individual P2Y receptors are identified.
Whereas P2X receptors convey rapid changes in the neuronal excitability on the millisecond timescale, P2Y receptors act on a longer, second timescale, appropriate for the fine-tuning of synaptic transmission. As for the signal transduction pathways activated by various subtypes of P2Y receptors, P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 receptors are coupled via Gq/11 proteins to stimulate phospholipase C, followed by increases in inositol phosphates and mobilization of Ca2+ from intracellular stores; in addition, the P2Y11 receptor mediates an increase in adenyl cyclase activity [84]. On the other hand, P2Y12, P2Y13, and P2Y14 receptors are coupled via Gi/o proteins to inhibit adenyl cyclase activity followed by a decrease in intracellular cAMP levels [84]. The activation of Gi protein-coupled P2Y receptors leads to the voltage-dependent inhibition of N-type voltage-sensitive Ca2+ channels directly or indirectly and subsequent inhibition of neurotransmitter release [85]. The inhibition of voltage-sensitive Ca2+ currents has also been demonstrated for those P2Y receptors, which are coupled to the Gq/11 proteins [85]; however, this inhibition is voltage independent [85]. In addition, the Gi/o-coupled P2Y receptors are known to activate voltage-sensitive GIRK K+ channels via direct interaction of Kir3 channel protein [85], which hyperpolarizes the neuronal membrane.
The distribution of P2Y receptors in neuronal structures
mRNA encoding all known P2Y receptors, i.e., P2Y1, P2Y2, P2Y4, P2Y6, P2Y12, P2Y13, and P2Y14 are present in the brain [81, 87–89]. Although our knowledge of their cell-specific localization at the protein level is still incomplete, it appears that a number of them, such as P2Y1, P2Y2, and P2Y6 receptors, are expressed both on neurons and astrocytes [90–96], whereas others are not exclusively, but predominantly localized to astrocytes (P2Y13: [97] P2Y14: [98]), oligodendrocytes (P2Y12: [99]], or microglia (P2Y12: [100]). P2Y receptor mRNA and protein can be detected in a number of different structures, including sympathetic and parasympathetic and sensory nerve terminals, basal ganglia, brainstem, cerebellum, cortex, hypothalamus, and hippocampus (for further references, see [85]). However, immunocytochemical data should be handled with caution due to the lack of verification of the specificity of many of the available antibodies. For detailed information on the distribution of individual P2Y receptor mRNAs and proteins, we refer to recent reviews on this particular topic [85, 101].
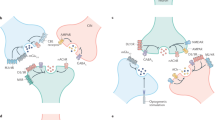
Modulation of neurotransmitter release by facilitatory P2 receptors
ACh
PNS
P2X receptors
Since P2X receptors have relatively high Ca2+ permeability [102, 103], this property makes them capable of initiating neurotransmitter release by Ca2+ influx through the receptor-ion channel complex or facilitating Ca2+-dependent neurotransmitter release, provided that they are located nearby the release sites (Table 1). The first report suggesting that P2 receptors are involved in the facilitatory modulation of neurotransmitter release stems from 1991 when we found that opposite to the well-known inhibitory action of adenosine, α,β-methylene ATP, a metabolically stable analogue of ATP, enhanced electrically evoked acetylcholine release from the myenteric plexus of guinea pig and facilitated the related contractile response [6]. This effect was not blocked by the antagonists of adenosine receptors, and therefore was proposed to be mediated by ATP-sensitive P2 receptors [6]. In the same time Fu and Poo observed that ATP potentiates the spontaneous secretion of acetylcholine from developing neuromuscular synapses in Xenopus cell culture by promoting Ca2+ influx through the plasma membrane. However, this effect was not recognized to be the result of P2 receptor activation [104]. Later on, P2 receptor-mediated facilitation of acetylcholine release was confirmed by electrophysiological recordings in chicken ciliary ganglion [105] and mouse motor nerve terminals [106]. Presynaptic P2X receptors, involved in the facilitation of acetylcholine release, have also been identified in developing and adult neuromuscular synapses of Xenopus [107] and rat [108]. Homomeric P2X7 receptors are inserted into the membrane of mouse motor nerve terminals and their activation elicits vesicular exocytosis [74, 109]. However, there is no report about the presence of other subunit compositions of P2X receptors at the neuromuscular junction and it is also unclear whether such facilitatory receptors also exist on the terminals of central cholinergic neurons.
Monoamines (NA, serotonin, DA)
PNS
P2X receptors
The presynaptic facilitatory action of ATP on noradrenergic transmission was described for the first time by Miyahara and Suzuki in rabbit ear artery [110]. It was followed by the demonstration of the facilitatory effect of ATP and its metabolically stable analogue α,β-methylene ATP on [3H]noradrenaline efflux in PC12 cells [111] and in the guinea pig ileum [6]. However, the knowledge on P2 receptors at that time did not allow the identification of P2 receptor subtypes involved in these effects. The issue has been reinvestigated and it was found that sympathetic nerve terminals are equipped with ionotropic P2X receptors, activation of which directly elicits or facilitates noradrenaline release elicited by nerve stimulation [1, 112–115] via a direct Ca2+ influx through the receptor-ion channel complex. The pharmacological phenotype of these receptors varies between species, between transmission sites of the sympathetic nervous system, and even between the somata and nerve terminals of an individual neuron. Thus, in cultured sympathetic neurons of the rat an α,β-methylene ATP-insensitive P2X2-like receptor was identified [116], whereas in the guinea pig right atrium we found that α,β-methylene ATP stimulates noradrenaline outflow and the pharmacological profile of the underlying receptor was similar to that of P2X3 or P2X2/P2X3 receptors, consistent with the expression of their mRNA in the sympathetic ganglia [115]. In another study the facilitatory P2X receptors involved in the modulation of noradrenaline outflow in the rat vas deferens were identified as P2X1, P2X3, or P2X2/P2X3 receptors [113]. In contrast, cultured mouse sympathetic nerve terminals do not seem to express facilitatory nucleotide-sensitive receptors [117]. Importantly, these receptors seem to be endogenously activated by ATP released in response to ongoing neuronal activity [115, 116] and by myocardial ischemia in the guinea pig [118], porcine, and human heart [119] and could contribute to ischemia-induced arrhythmia and ischemic heart dysfunction.
CNS
P2X receptors
In the central nervous system, locus coeruleus (LC) neurons of the rat are equipped with ATP-sensitive P2X-like receptors, which facilitate the discharge of spontaneous action potentials [120]. Facilitatory P2X receptors have also been described in the noradrenergic axon terminals innervating the hippocampus, and the homomeric P2X1 and P2X3 receptors were identified as the most likely subunits responsible for this action [68].
P2Y receptors
P2 receptors enhance the release of serotonin from the hippocampus [121] and that of dopamine from the striatum [122, 123], and the latter effects are thought to be mediated by P2Y receptors. However, the pre- or postsynaptic localization of receptors responsible for these effects were not clarified in these studies. The P2 receptor agonist 2-methyl-thio ATP releases dopamine from the nucleus accumbens through direct and indirect mechanisms [124–126] in vivo. Interestingly, P2 receptor activation-evoked dopamine release seems to play a role in the modulation of feeding behavior as P2 receptor antagonists inhibit feeding-induced dopamine release and concomitant behavioral changes after food deprivation [127, 128].
Excitatory amino acids (glutamate, aspartate)
CNS
P2X receptors
In addition to ACh and monoamines, the release of excitatory amino acid transmitters is also modulated by presynaptic P2X receptors in the CNS, as demonstrated partly by neurochemical and partly by electrophysiological methods.
Activation of P2X receptors elicits glutamate release in the brainstem [129–132], hippocampus [69, 75, 133–135], and cortical synaptosomes [136]. As for the underlying receptor subunits involved in these effects, P2X1 [69], P2X2 [133], P2X3, and P2X2/3 receptors [69, 130] as well as P2X7 [75, 132, 134, 136, 137] were identified. The involvement of P2X2 receptors [133] and P2X7 receptors [137] has been confirmed by the use of transgenic mice deficient in P2X2 and P2X7 receptors, respectively. Moreover the activation of P2X7 receptors not only elicits glutamate release but also permits the activation of other ligand-gated ion channels on the nerve terminals, such as α7 nicotinic receptors, as demonstrated recently in rat cortical synaptosomes [136]. The activation of a P2X7-like receptor promotes Ca2+ influx in cortical synaptosomes [138] and in isolated midbrain synaptic terminals [139] and activates p38MAP kinase enzyme in the hippocampus [140]. This latter effect seems to participate in the effect of ATP to elicit glutamate release as it was sensitive to the inhibition by the specific p38MAP kinase inhibitor, SB203580 [140]. Nevertheless, the exact mechanism whereby the P2X7 receptor and subsequent activation of p38MAP kinase enzyme leads to increased glutamate release awaits further investigation. In addition, it has been reported that the P2X7 receptor agonist BzATP depresses synaptic transmission at the mossy fiber-CA3 synapse [141] in a p38MAPK-dependent way. However, more recently the participation of P2X7 receptors in this latter effect has been disproved [76, 77]. In addition to nerve terminals, P2X7 receptor activation also elicits glutamate release from cultured astrocytes [142] and inhibits the uptake of glutamate in Müller glial cells of the retina [143].
The activation of P2X receptors facilitates excitatory transmission in the spinal cord, releasing glutamate from primary afferent fibers terminating in lamina II [144–148] and lamina V [144]; these actions are mediated by P2X3, P2X1/5, and P2X4/6 receptors, respectively. Finally, the ability of the P2 receptor ligand 2-methyl-thio ATP to release glutamate has also been demonstrated in vivo in the dopamine-depleted nucleus accumbens [149], although the underlying receptor subtype was not identified in this study.
P2Y receptors
Interestingly, the activation of P2Y receptors is also implicated to elicit and potentiate glutamate release in the central nervous system. In the medial habenula nucleus UDP and UTP increase presynaptic release probability and elicit a non-Hebbian-type long-term potentiation of excitatory transmission, an effect probably mediated by P2Y4 receptors [150]. In addition, the activation of P2Y1 receptors elicits vesicular glutamate release from astrocytes [151] and from cultured Schwann cells [152].
Inhibitory amino acids (GABA, glycine)
CNS
P2X receptors
ATP or P1,P5-di(adenosine-5′) pentaphosphate (Ap5A) elicits an increase in the intrasynaptosomal calcium and induces subsequent GABA release in midbrain GABAergic synaptosomes via activation of P2X3 and a dinucleotide receptor [153]. The regulation of GABA release by P2X receptors has also been reported in the spinal cord [154], cultured cortical [155] and hippocampal [156] cells, and the brainstem, where the excitatory and inhibitory synaptic transmission is facilitated via P2X3 and P2X1 receptors, respectively [157]. In addition to direct modulation of glutamate release, P2X7 receptor activation also releases GABA from the hippocampus through the activation on non-NMDA-type glutamate receptors [75]. This effect is absent in mice genetically deficient in P2X7 receptors [137] and mediated by the sodium-dependent reversal of the GABA transporter [75]. P2X receptor-mediated, TTX-sensitive GABA release has been implicated in the accelerated recovery of guinea pig hippocampal slices from a hypoxic/hypoglycemic insult [158]. The activation of P2X7 receptors also releases GABA from cultured RBA astrocytes, however, with a different mechanism, by participation of the HCO3 -/Cl- exchanger [159]. On the other hand, no evidence was found for a direct facilitation of GABA release by P2 receptors in the hippocampal nerve terminal preparation [160]. Nevertheless, the release of another inhibitory transmitter, glycine, is augmented by P2X receptor activation in the dorsal horn [161] and in the brainstem trigeminal nucleus [162].
P2Y receptors
In addition to P2X receptors, activation of P2Y1 receptors leads to an increase of the inhibitory postsynaptic current (IPSC) frequency in an acute hippocampal slice in a manner dependent on action potential generation, indicating that this effect is related to the activation of receptors present on the somata/dendrites of hippocampal interneurons [163].
Modulation of neurotransmitter release by inhibitory P2 receptors
In addition to facilitatory modulation, P2 receptors are also involved in the inhibitory modulation of the release of various transmitters and the metabotropic P2Y receptors are thought to play a major role in these actions (Table 2).
ACh
PNS
It has been known for a long time that ATP is involved in the inhibitory presynaptic modulation of cholinergic transmission [164]. However, it has been the subject of a long-standing debate whether ATP itself is responsible for this effect or its degradation product adenosine [165–167], whereas an alternative was that ATP itself acts on adenosine receptors [168] or activates a putative P3 receptor bearing pharmacological features of both P1 and P2 receptors [169]. A more definitive proof for the involvement of P2 receptors in the inhibition of acetylcholine release was obtained later in the frog neuromuscular junction [170], rat submandibular ganglia [171], and rabbit retina [172]. Presynaptic P2Y receptors responsible for the inhibition of spontaneous acetylcholine release were recently identified at the mouse neuromuscular junction [173]. In this study the underlying subcellular mechanism of the inhibition of acetylcholine release was also explored: the activation of P2Y receptors is coupled to Gi/o proteins and modulates presynaptic Ca2+ channels related to tonic secretion of acetylcholine [173].
CNS
Inhibitory P2 receptors involved in the modulation of ACh release have been demonstrated in rat cerebral cortex [47]. By contrast, in the hippocampus, ATP primarily inhibits acetylcholine release through its breakdown to adenosine and subsequent action on A1 adenosine receptors [47].
Monoamines (NA, serotonin, DA)
PNS
The presence of nucleotide-sensitive inhibitory P2 receptors on postganglionic sympathetic neurons was recognized relatively early [5, 174–179], although initially these receptors were qualified as P2Y-like [5] or as putative P3 receptors, which are “hybrid” receptors between P1 and P2 receptors and sensitive to adenine nucleotides but also to theophylline derivatives [176–178]. These receptors have been described and characterized in sympathetic nerves innervating the rat caudal artery [176, 177, 180], guinea pig saphenous artery [175], vas deferens [5, 178, 179, 181, 182], atrium [183], iris [184], kidney [185], and pancreas [186] as well as in cultured sympathetic neurons [187, 188]. As for subtype-specific identification, Queiroz et al. [113] identified presynaptic inhibitory nucleotide receptors on the noradrenergic axon terminals of the rat vas deferens as P2Y12 and/or P2Y13 receptors, whereas on cultured sympathetic neurons [189] and bovine adrenal chromaffin cells [190] only P2Y12 receptors have been identified. Interestingly, it appears that mouse sympathetic neurons [191] and noradrenergic nerves innervating the rat adrenal cortex [192] do not express an inhibitory P2 receptor. The mechanism of P2Y receptor-mediated inhibition of noradrenaline release has also been explored in several studies: the activation of P2Y receptors inhibits voltage-dependent Ca2+ influx and thereby limits the Ca2+-dependent vesicular exocytosis and subsequent efflux of noradrenaline to the extracellular space [189, 193, 194].
CNS
Similar inhibitory P2Y receptors have also been reported in the CNS in the rat brain cortex [195] and hippocampus [196]; however P2Y receptor subtypes involved were not identified in these early studies.
In the CNS, ATP inhibits the release of serotonin [197] and dopamine [198] via activation of metabotropic P2 receptors.
Excitatory amino acids (glutamate, aspartate)
CNS
ATP and its metabolically stable analogue ATP-γ-S inhibits depolarization-evoked glutamate release from rat brain cortex slices [199] and inhibits glutamatergic EPSPs in hippocampal CA1 synapses [200]. Although the underlying receptor was sensitive to theophylline derivatives, the authors proposed that ATP acted through a putative pertussis toxin-sensitive P2Y receptor. However, this hypothesis was challenged by showing the rapid and highly effective hydrolysis of ATP in the hippocampal slices [45, 46] and by the demonstration of the complete absence of nucleotide-mediated modulation of excitatory synaptic transmission in the hippocampi of A1 receptor-/- mice [201]. In a recent study Rodrigues et al. demonstrated that single hippocampal pyramidal neurons do express P2Y1, P2Y2, and P2Y4 receptors, and the release of glutamate, measured by a neurochemical technique, is inhibited by these receptors [69]. The discrepancy between the observations obtained in electrophysiological and neurochemical studies might be explained by the fact that in the former, individual synapses, whereas in the latter, glutamate release from all synapses of the hippocampal slice were simultaneously investigated. Nevertheless, the exact conditions under which the activation of P2Y receptors by endogenous ligands gain significance remain to be identified.
Functional data suggest that the release of glutamate in the spinal cord is modulated by inhibitory P2Y receptors. The activation of P2Y receptors causes blockade of the N-type calcium channels in dorsal root ganglion (DRG) cells [202], and this effect may decrease the release of glutamate from DRG terminals in the spinal cord and thereby partly counterbalance the algogenic effect of ATP [203, 204]. This assumption is supported by the findings that the P2Y1/12/13 receptor agonist ADP-β-S inhibits polysynaptic, but not monosynaptic excitatory postsynaptic potentials in the hemisected spinal cord and exhibits antinociceptive potential in the tail flick test [204].
Recent studies revealed that modulators released from glial cells also regulate neurotransmitter release from nearby nerve terminals by the activation of P2 receptors. Hence mechanical stimulation of astrocytes in hippocampal cell culture leads to the generation of Ca2+ waves in astrocytes, which spread by the release of ATP and subsequent activation of P2 receptors and lead to the depression of excitatory synaptic transmission between neurons [205]. This glia-driven synaptic depression is partly mediated by ATP itself acting on P2Y receptors and partly by adenosine acting on A1 adenosine receptors [205]. A similar mechanism has also been demonstrated in intact hippocampal slices, where ATP released from neurons and astrocytes acts on P2Y1 receptors to excite interneurons, resulting in increased synaptic inhibition within intact hippocampal circuits [206]. On the other hand, to our knowledge there is no information regarding whether the release of GABA and other inhibitory amino acids is subject to modulation via inhibitory P2 receptors.
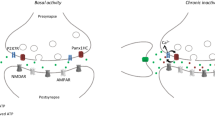
Potential therapeutic utilization of P2 receptors involved in the regulation of neurotransmitter release
P2X and P2Y receptors involved in the regulation of neurotransmitter release offer attractive, although not yet utilized sites for pharmacotherapy in nervous system diseases. For instance, facilitatory P2X receptors present on axon terminals could be activated not only during normal neuronal activity, but also during pathological situations, when cellular damage provides an ATP-rich extracellular milieu nearby the receptors. Thus, P2X receptors present on the sympathetic nerve terminals supplying the heart seem to be endogenously activated by ATP by myocardial ischemia [118, 119] and could contribute to ischemia-induced arrhythmia and ischemic heart dysfunction. Therefore, inhibition of these facilitatory P2X receptors might have therapeutic relevance in ischemic heart disease. The pathological activation of CNS P2X receptors, regulating the release of glutamate during ischemic-like conditions, was also recently described [207]. Increased activation of P2X receptors could contribute to ischemia-evoked glutamate release and thereby to glutamatergic excitotoxicity and resultant neuronal death; therefore, inhibition of these receptors could be a promising approach to treat ischemia-related neurodegenerative diseases. An analogous mechanism could play a role in the spinal cord during the sensitization process leading to various forms of sensory neuropathy; therefore, attenuation of increased glutamate release from the central terminals of primary sensory neurons by the inhibition of P2X receptors is a potential pathway which could be utilized in neuropathic pain. Since inhibitory P2Y receptors are frequently coexpressed on nerve terminals that are equipped with P2X receptors, activation of P2Y receptors could have a similar effect as the inhibition of P2X receptors. Therefore P2Y receptor agonists might also have therapeutic value in the areas described above. However, one should bear in mind that different subtypes of P2X and P2Y receptors affect various other aspects of physiological and pathological neuronal functions, which could also modify their potential.
Conclusion
In conclusion, substantial advances have been obtained in the identification and characterization of neurotransmitter release modulating P2 receptors in recent years. It appears that almost all major neurotransmitters of the nervous system are subject to neuromodulation by nucleotide-sensitive P2 receptors. Although there are exceptions to this rule, in general the release of different transmitters is subject to a dual modulation similar to modulation of other transmitters of the CNS and PNS: facilitatory modulation is conveyed by ionotropic P2X receptors, whereas inhibitory modulation is mediated by G protein-coupled metabotropic P2Y receptors. Amongst P2X receptors, P2X1, P2X2, P2X3, P2X2/3, P2X1/5, P2X4/6, and P2X7 receptors were identified to be responsible for facilitatory modulation in different areas of the CNS and PNS. In addition, P2Y receptors (P2Y1, P2Y4) could also mediate facilitation of transmitter release in certain areas. Inhibitory modulation of neurotransmitter release is mediated by P2Y12 and P2Y13 receptors; however, individual P2Y receptor subtypes involved in these interactions are far from fully explored yet. It appears that not only neuronal, but also glia-derived ATP play a role in the modulation of neurotransmitter release. The intensity of P2 receptor-mediated modulation, the balance between the facilitatory and inhibitory modulation and the participating individual receptor subtypes, however, varies between individual transmission sites, depending on the expression pattern of P2 receptors and the factors determining the nucleotide levels in the vicinity of release of modulatory P2 receptors. Therefore, further progress is necessary in order to obtain a precise mapping of P2 receptor-mediated modulation of neurotransmitter release. The in vivo relevance of most of the in vitro observations on presynaptic P2 receptors awaits further investigation. Finally, physiological and pathological situations where presynaptic P2 receptors become endogenously activated by released nucleotides need to be identified.
Abbreviations
- ABC:
-
ATP binding cassette
- ACh:
-
acetylcholine
- AP:
-
action potential
- Ap5A:
-
P1,P5-di(adenosine-5′) pentaphosphate
- BzATP:
-
benzoylbenzoylATP
- CNS:
-
central nervous system
- DA:
-
dopamine
- DRG:
-
dorsal root ganglion
- ENPP:
-
ectonucleotide pyrophosphatase
- EJP:
-
excitatory junction potential
- ENTPDase:
-
ectonucleoside triphosphate diphosphohydrolase
- EPP:
-
end plate potential
- EPSC:
-
excitatory postsynaptic current
- EPSP:
-
excitatory postsynaptic potential
- GABA:
-
γ-aminobutyric acid
- GPCR:
-
G-protein coupled receptor
- IL-1β:
-
interleukin-1β
- IPSC:
-
inhibitory postsynaptic current
- LC:
-
locus coeruleus
- LPS:
-
lipopolysaccharide
- mEPP:
-
miniature EPP
- mEPSC:
-
miniature EPSC
- NA:
-
noradrenaline
- NMJ:
-
neuromuscular junction
- NT:
-
neurotransmitter
- NTS:
-
nucleus tractus solitarii
- PNS:
-
peripheral nervous system
- sEPP:
-
spontaneous EPP
- sIPSC:
-
spontaneous IPSC
- UDP:
-
uridine 5′-diphosphate
- UTP:
-
uridine 5′-triphosphate
References
Boehm S, Kubista H (2002) Fine tuning of sympathetic transmitter release via ionotropic and metabotropic presynaptic receptors. Pharmacol Rev 54:43–99
McGehee DS, Role LW (1996) Presynaptic ionotropic receptors. Curr Opin Neurobiol 6:342–349
Vizi ES (2000) Role of high-affinity receptors and membrane transporters in nonsynaptic communication and drug action in the central nervous system. Pharmacol Rev 52:63–89
MacDermott AB, Role LW, Siegelbaum SA (1999) Presynaptic ionotropic receptors and the control of transmitter release. Annu Rev Neurosci 22:443–485
von Kugelgen I, Schoffel E, Starke K (1989) Inhibition by nucleotides acting at presynaptic P2-receptors of sympathetic neuro-effector transmission in the mouse isolated vas deferens. Naunyn Schmiedebergs Arch Pharmacol 340:522–532
Sperlagh B, Vizi ES (1991) Effect of presynaptic P2 receptor stimulation on transmitter release. J Neurochem 56:1466–1470
Cunha RA, Ribeiro JA (2000) ATP as a presynaptic modulator. Life Sci 68:119–137
Sperlágh B, Vizi ES (1996) Neuronal synthesis, storage and release of ATP. Semin Neurosci 8:175–186
Sperlagh B, Vizi ES (2000) Regulation of purine release. In: Abbracchio MP, Williams M (eds) Handbook of experimental pharmacology, vol 151. Springer, Berlin, pp 179–209
Cunha RA, Vizi ES, Ribeiro JA et al (1996) Preferential release of ATP and its extracellular catabolism as a source of adenosine upon high- but not low-frequency stimulation of rat hippocampal slices. J Neurochem 67:2180–2187
Sperlagh B, Kittel A, Lajtha A et al (1995) ATP acts as fast neurotransmitter in rat habenula: neurochemical and enzymecytochemical evidence. Neuroscience 66:915–920
Wieraszko A, Goldsmith G, Seyfried TN (1989) Stimulation-dependent release of adenosine triphosphate from hippocampal slices. Brain Res 485:244–250
Braun N, Sevigny J, Robson SC et al (2000) Assignment of ecto-nucleoside triphosphate diphosphohydrolase-1/cd39 expression to microglia and vasculature of the brain. Eur J Neurosci 12:4357–4366
Zimmermann H (2000) Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol 362:299–309
Coco S, Calegari F, Pravettoni E et al (2003) Storage and release of ATP from astrocytes in culture. J Biol Chem 278:1354–1362
Wang X, Arcuino G, Takano T et al (2004) P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med 10:821–827
Verderio C, Matteoli M (2001) ATP mediates calcium signaling between astrocytes and microglial cells: modulation by IFN-gamma. J Immunol 166:6383–6391
Gourine AV, Llaudet E, Dale N et al (2005) ATP is a mediator of chemosensory transduction in the central nervous system. Nature 436:108–111
Wang Y, Roman R, Lidofsky SD et al (1996) Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc Natl Acad Sci U S A 93:12020–12025
Melani A, Turchi D, Vannucchi MG et al (2005) ATP extracellular concentrations are increased in the rat striatum during in vivo ischemia. Neurochem Int 47:442–448
Lutz PL, Kabler S (1997) Release of adenosine and ATP in the brain of the freshwater turtle (Trachemys scripta) during long-term anoxia. Brain Res 769:281–286
Juranyi Z, Sperlagh B, Vizi ES (1999) Involvement of P2 purinoceptors and the nitric oxide pathway in [3H]purine outflow evoked by short-term hypoxia and hypoglycemia in rat hippocampal slices. Brain Res 823:183–190
Hisanaga K, Onodera H, Kogure K (1986) Changes in levels of purine and pyrimidine nucleotides during acute hypoxia and recovery in neonatal rat brain. J Neurochem 47:1344–1350
Frenguelli BG, Wigmore G, Llaudet E et al (2007) Temporal and mechanistic dissociation of ATP and adenosine release during ischaemia in the mammalian hippocampus. J Neurochem 101:1400–1413
Gourine AV, Llaudet E, Dale N et al (2005) Release of ATP in the ventral medulla during hypoxia in rats: role in hypoxic ventilatory response. J Neurosci 25:1211–1218
Ferrari D, Chiozzi P, Falzoni S et al (1997) Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J Exp Med 185:579–582
Sperlagh B, Hasko G, Nemeth Z et al (1998) ATP released by LPS increases nitric oxide production in raw 264.7 macrophage cell line via P2Z/P2X7 receptors. Neurochem Int 33:209–215
Sperlagh B, Baranyi M, Hasko G et al (2004) Potent effect of interleukin-1 beta to evoke ATP and adenosine release from rat hippocampal slices. J Neuroimmunol 151:33–39
Sperlagh B, Szabo G, Erdelyi F et al (2003) Homo- and heteroexchange of adenine nucleotides and nucleosides in rat hippocampal slices by the nucleoside transport system. Br J Pharmacol 139:623–633
Latini S, Pedata F (2001) Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem 79:463–484
Pearson RA, Dale N, Llaudet E et al (2005) ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron 46:731–744
Zhang X, Chen Y, Wang C et al (2007) Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci U S A 104:9864–9869
Schwiebert EM (1999) ABC transporter-facilitated ATP conductive transport. Am J Physiol 276:C1–C8
al-Awqati Q (1995) Regulation of ion channels by ABC transporters that secrete ATP. Science 269:805–806
Ballerini P, Di Iorio P, Ciccarelli R et al (2002) Glial cells express multiple ATP binding cassette proteins which are involved in ATP release. Neuroreport 13:1789–1792
Abdipranoto A, Liu GJ, Werry EL et al (2003) Mechanisms of secretion of ATP from cortical astrocytes triggered by uridine triphosphate. Neuroreport 14:2177–2181
Darby M, Kuzmiski JB, Panenka W et al (2003) ATP released from astrocytes during swelling activates chloride channels. J Neurophysiol 89:1870–1877
Stout C, Goodenough DA, Paul DL (2004) Connexins: functions without junctions. Curr Opin Cell Biol 16:507–512
Stout CE, Costantin JL, Naus CC et al (2002) Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem 277:10482–10488
Gomes P, Srinivas SP, Van Driessche W et al (2005) ATP release through connexin hemichannels in corneal endothelial cells. Invest Ophthalmol Vis Sci 46:1208–1218
Braet K, Vandamme W, Martin PE et al (2003) Photoliberating inositol-1,4,5-trisphosphate triggers ATP release that is blocked by the connexin mimetic peptide gap 26. Cell Calcium 33:37–48
Cook SP, McCleskey EW (2002) Cell damage excites nociceptors through release of cytosolic ATP. Pain 95:41–47
Wang TF, Rosenberg PA, Guidotti G (1997) Characterization of brain ecto-apyrase: evidence for only one ecto-apyrase (CD39) gene. Brain Res Mol Brain Res 47:295–302
Wang TF, Guidotti G (1998) Widespread expression of ecto-apyrase (CD39) in the central nervous system. Brain Res 790:318–322
Cunha RA, Sebastiao AM, Ribeiro JA (1998) Inhibition by ATP of hippocampal synaptic transmission requires localized extracellular catabolism by ecto-nucleotidases into adenosine and channeling to adenosine A1 receptors. J Neurosci 18:1987–1995
Dunwiddie TV, Diao L, Proctor WR (1997) Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J Neurosci 17:7673–7682
Cunha RA, Ribeiro JA, Sebastiao AM (1994) Purinergic modulation of the evoked release of [3H]acetylcholine from the hippocampus and cerebral cortex of the rat: role of the ectonucleotidases. Eur J Neurosci 6:33–42
Khakh BS (2001) Molecular physiology of P2X receptors and ATP signalling at synapses. Nat Rev Neurosci 2:165–174
Valera S, Hussy N, Evans RJ et al (1994) A new class of ligand-gated ion channel defined by P2X receptor for extracellular ATP. Nature 371:516–519
North RA, Surprenant A (2000) Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol 40:563–580
Torres GE, Egan TM, Voigt MM (1999) Hetero-oligomeric assembly of P2X receptor subunits. Specificities exist with regard to possible partners. J Biol Chem 274:6653–6659
Jones CA, Vial C, Sellers LA et al (2004) Functional regulation of P2X6 receptors by N-linked glycosylation: identification of a novel alpha beta-methylene ATP-sensitive phenotype. Mol Pharmacol 65:979–985
Gever JR, Cockayne DA, Dillon MP et al (2006) Pharmacology of P2X channels. Pflugers Arch 452:513–537
Nicke A, Kerschensteiner D, Soto F (2005) Biochemical and functional evidence for heteromeric assembly of P2X1 and P2X4 subunits. J Neurochem 92:925–933
Le KT, Babinski K, Seguela P (1998) Central P2X4 and P2X6 channel subunits coassemble into a novel heteromeric ATP receptor. J Neurosci 18:7152–7159
Lewis C, Neidhart S, Holy C et al (1995) Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature 377:432–435
Brown SG, Townsend-Nicholson A, Jacobson KA et al (2002) Heteromultimeric P2X(1/2) receptors show a novel sensitivity to extracellular pH. J Pharmacol Exp Ther 300:673–680
King BF, Townsend-Nicholson A, Wildman SS et al (2000) Coexpression of rat P2X2 and P2X6 subunits in Xenopus oocytes. J Neurosci 20:4871–4877
Torres GE, Haines WR, Egan TM et al (1998) Co-expression of P2X1 and P2X5 receptor subunits reveals a novel ATP-gated ion channel. Mol Pharmacol 54:989–993
Collo G, North RA, Kawashima E et al (1996) Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci 16:2495–2507
Atkinson L, Batten TF, Deuchars J (2000) P2X2 receptor immunoreactivity in the dorsal vagal complex and area postrema of the rat. Neuroscience 99:683–696
Rubio M, Soto F (2001) Distinct localization of P2X receptors at excitatory postsynaptic specializations. J Neurosci 21:641–653
Vulchanova L, Arvidsson U, Riedl M et al (1996) Differential distribution of two ATP-gated channels (P2X receptors) determined by immunocytochemistry. Proc Natl Acad Sci U S A 93:8063–8067
Kanjhan R, Housley GD, Burton LD et al (1999) Distribution of the P2X2 receptor subunit of the ATP-gated ion channels in the rat central nervous system. J Comp Neurol 407:11–32
Vulchanova L, Riedl MS, Shuster SJ et al (1997) Immunohistochemical study of the P2X2 and P2X3 receptor subunits in rat and monkey sensory neurons and their central terminals. Neuropharmacology 36:1229–1242
Calvert JA, Evans RJ (2004) Heterogeneity of P2X receptors in sympathetic neurons: contribution of neuronal P2X1 receptors revealed using knockout mice. Mol Pharmacol 65:139–148
Knott TK, Velazquez-Marrero C, Lemos JR (2005) ATP elicits inward currents in isolated vasopressinergic neurohypophysial terminals via P2X2 and P2X3 receptors. Pflugers Arch 450:381–389
Papp L, Balazsa T, Kofalvi A et al (2004) P2X receptor activation elicits transporter-mediated noradrenaline release from rat hippocampal slices. J Pharmacol Exp Ther 310:973–980
Rodrigues RJ, Almeida T, Richardson PJ et al (2005) Dual presynaptic control by ATP of glutamate release via facilitatory P2X1 P2X2/3, and P2X3 and inhibitory P2Y1, P2Y2, and/or P2Y4 receptors in the rat hippocampus. J Neurosci 25:6286–6295
Le KT, Paquet M, Nouel D et al (1997) Primary structure and expression of a naturally truncated human P2X ATP receptor subunit from brain and immune system. FEBS Lett 418:195–199
Yao ST, Barden JA, Lawrence AJ (2001) On the immunohistochemical distribution of ionotropic P2X receptors in the nucleus tractus solitarius of the rat. Neuroscience 108:673–685
Collo G, Neidhart S, Kawashima E et al (1997) Tissue distribution of the P2X7 receptor. Neuropharmacology 36:1277–1283
Atkinson L, Batten TF, Moores TS et al (2004) Differential co-localisation of the P2X7 receptor subunit with vesicular glutamate transporters VGLUT1 and VGLUT2 in rat CNS. Neuroscience 123:761–768
Deuchars SA, Atkinson L, Brooke RE et al (2001) Neuronal P2X7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems. J Neurosci 21:7143–7152
Sperlagh B, Kofalvi A, Deuchars J, Atkinson L et al (2002) Involvement of P2X7 receptors in the regulation of neurotransmitter release in the rat hippocampus. J Neurochem 81:1196–1211
Sim JA, Young MT, Sung HY et al (2004) Reanalysis of P2X7 receptor expression in rodent brain. J Neurosci 24:6307–6314
Kukley M, Stausberg P, Adelmann G et al (2004) Ecto-nucleotidases and nucleoside transporters mediate activation of adenosine receptors on hippocampal mossy fibers by P2X7 receptor agonist 2′-3′-O-(4-benzoylbenzoyl)-ATP. J Neurosci 24:7128–7139
Sanchez-Nogueiro J, Marin-Garcia P, Miras-Portugal MT (2005) Characterization of a functional P2X7-like receptor in cerebellar granule neurons from P2X7 knockout mice. FEBS Lett 579:3783–3788
Abbracchio MP, Boeynaems JM, Barnard EA et al (2003) Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci 24:52–55
Barnard EA, Simon J (2001) An elusive receptor is finally caught: P2Y(12′), an important drug target in platelets. Trends Pharmacol Sci 22:388–391
Communi D, Gonzalez NS, Detheux M et al (2001) Identification of a novel human ADP receptor coupled to G(i). J Biol Chem 276:41479–41485
von Kugelgen I, Wetter A (2000) Molecular pharmacology of P2Y-receptors. Naunyn Schmiedebergs Arch Pharmacol 362:310–323
Abbracchio MP, Burnstock G, Boeynaems JM et al (2006) International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58:281–341
von Kugelgen I (2006) Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther 110:415–432
Hussl S, Boehm S (2006) Functions of neuronal P2Y receptors. Pflugers Arch 452:538–551
Yoshioka K, Saitoh O, Nakata H (2001) Heteromeric association creates a P2Y-like adenosine receptor. Proc Natl Acad Sci U S A 98:7617–7622
Chambers JK, Macdonald LE, Sarau HM et al (2000) A G protein-coupled receptor for UDP-glucose. J Biol Chem 275:10767–10771
Hollopeter G, Jantzen HM, Vincent D et al (2001) Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 409:202–207
Nicholas RA (2001) Identification of the P2Y12 receptor: a novel member of the P2Y family of receptors activated by extracellular nucleotides. Mol Pharmacol 60:416–420
Moore DJ, Chambers JK, Wahlin JP et al (2001) Expression pattern of human P2Y receptor subtypes: a quantitative reverse transcription-polymerase chain reaction study. Biochim Biophys Acta 1521:107–119
Moran-Jimenez MJ, Matute C (2000) Immunohistochemical localization of the P2Y1 purinergic receptor in neurons and glial cells of the central nervous system. Brain Res Mol Brain Res 78:50–58
Franke H, Krugel U, Grosche J et al (2004) P2Y receptor expression on astrocytes in the nucleus accumbens of rats. Neuroscience 127:431–441
Zhu Y, Kimelberg HK (2004) Cellular expression of P2Y and beta-AR receptor mRNAs and proteins in freshly isolated astrocytes and tissue sections from the CA1 region of P8-12 rat hippocampus. Brain Res 148:77–87
Loesch A, Glass R (2006) Electron microscopy and in situ hybridization: expression of P2Y2 receptor mRNA in the cerebellum. Methods Mol Biol (Clifton, NJ) 326:151–162
Sergeeva OA, Klyuch BP, Fleischer W et al (2006) P2Y receptor-mediated excitation in the posterior hypothalamus. Eur J Neurosci 24:1413–1426
Calvert JA, Atterbury-Thomas AE, Leon C et al (2004) Evidence for P2Y1, P2Y2, P2Y6 and atypical UTP-sensitive receptors coupled to rises in intracellular calcium in mouse cultured superior cervical ganglion neurons and glia. Br J Pharmacol 143:525–532
Fumagalli M, Trincavelli L, Lecca D et al (2004) Cloning, pharmacological characterisation and distribution of the rat G-protein-coupled P2Y13 receptor. Biochem Pharmacol 68:113–124
Moore DJ, Murdock PR, Watson JM et al (2003) GPR105, a novel Gi/o-coupled UDP-glucose receptor expressed on brain glia and peripheral immune cells, is regulated by immunologic challenge: possible role in neuroimmune function. Brain Res 118:10–23
Amadio S, Tramini G, Martorana A et al (2006) Oligodendrocytes express P2Y12 metabotropic receptor in adult rat brain. Neuroscience 141:1171–1180
Haynes SE, Hollopeter G, Yang G et al (2006) The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci 9:1512–1519
Burnstock G, Knight GE (2004) Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol 240:31–304
Egan TM, Khakh BS (2004) Contribution of calcium ions to P2X channel responses. J Neurosci 24:3413–3420
Rogers M, Colquhoun LM, Patrick JW et al (1997) Calcium flux through predominantly independent purinergic ATP and nicotinic acetylcholine receptors. J Neurophysiol 77:1407–1417
Fu WM, Poo MM (1991) ATP potentiates spontaneous transmitter release at developing neuromuscular synapses. Neuron 6:837–843
Sun XP, Stanley EF (1996) An ATP-activated, ligand-gated ion channel on a cholinergic presynaptic nerve terminal. Proc Natl Acad Sci U S A 93:1859–1863
Hong SJ, Chang CC (1998) Evaluation of intrinsic modulation of synaptic transmission by ATP in mouse fast twitch muscle. J Neurophysiol 80:2550–2558
Fu WM, Chen YH, Lee KF et al (1997) Regulation of quantal transmitter secretion by ATP and protein kinases at developing neuromuscular synapses. Eur J Neurosci 9:676–685
Salgado AI, Cunha RA, Ribeiro JA (2000) Facilitation by P2 receptor activation of acetylcholine release from rat motor nerve terminals: interaction with presynaptic nicotinic receptors. Brain Res 877:245–250
Moores TS, Hasdemir B, Vega-Riveroll L et al (2005) Properties of presynaptic P2X7-like receptors at the neuromuscular junction. Brain Res 1034:40–50
Miyahara H, Suzuki H (1987) Pre- and post-junctional effects of adenosine triphosphate on noradrenergic transmission in the rabbit ear artery. J Physiol 389:423–440
Inoue K, Nakazawa K, Fujimori K et al (1989) Extracellular adenosine 5′-triphosphate-evoked norepinephrine secretion not relating to voltage-gated Ca channels in pheochromocytoma PC12 cells. Neurosci Lett 106:294–299
Arthur DB, Taupenot L, Insel PA (2007) Nerve growth factor-stimulated neuronal differentiation induces changes in P2 receptor expression and nucleotide-stimulated catecholamine release. J Neurochem 100:1257–1264
Queiroz G, Talaia C, Goncalves J (2003) ATP modulates noradrenaline release by activation of inhibitory P2Y receptors and facilitatory P2X receptors in the rat vas deferens. J Pharmacol Exp Ther 307:809–815
Sesti C, Broekman MJ, Drosopoulos JH et al (2002) EctoNucleotidase in cardiac sympathetic nerve endings modulates ATP-mediated feedback of norepinephrine release. J Pharmacol Exp Ther 300:605–611
Sperlagh B, Erdelyi F, Szabo G et al (2000) Local regulation of [3H]-noradrenaline release from the isolated guinea-pig right atrium by P2X-receptors located on axon terminals. Br J Pharmacol 131:1775–1783
Boehm S (1999) ATP stimulates sympathetic transmitter release via presynaptic P2X purinoceptors. J Neurosci 19:737–746
Norenberg W, Gobel I, Meyer A et al (2001) Stimulation of mouse cultured sympathetic neurons by uracil but not adenine nucleotides. Neuroscience 103:227–236
Sesti C, Koyama M, Broekman MJ et al (2003) Ectonucleotidase in sympathetic nerve endings modulates ATP and norepinephrine exocytosis in myocardial ischemia. J Pharmacol Exp Ther 306:238–244
Machida T, Heerdt PM, Reid AC et al (2005) Ectonucleoside triphosphate diphosphohydrolase 1/CD39, localized in neurons of human and porcine heart, modulates ATP-induced norepinephrine exocytosis. J Pharmacol Exp Ther 313:570–577
Frohlich R, Boehm S, Illes P (1996) Pharmacological characterization of P2 purinoceptor types in rat locus coeruleus neurons. Eur J Pharmacol 315:255–261
Okada M, Kawata Y, Murakami T et al (1999) Interaction between purinoceptor subtypes on hippocampal serotonergic transmission using in vivo microdialysis. Neuropharmacology 38:707–715
Zhang YX, Yamashita H, Ohshita T et al (1996) ATP induces release of newly synthesized dopamine in the rat striatum. Neurochem Int 28:395–400
Zhang YX, Yamashita H, Ohshita T et al (1995) ATP increases extracellular dopamine level through stimulation of P2Y purinoceptors in the rat striatum. Brain Res 691:205–212
Krugel U, Kittner H, Illes P (1999) Adenosine 5′-triphosphate-induced dopamine release in the rat nucleus accumbens in vivo. Neurosci Lett 265:49–52
Krugel U, Kittner H, Illes P (2001) Mechanisms of adenosine 5′-triphosphate-induced dopamine release in the rat nucleus accumbens in vivo. Synapse 39:222–232
Krugel U, Kittner H, Franke H et al (2003) Purinergic modulation of neuronal activity in the mesolimbic dopaminergic system in vivo. Synapse 47:134–142
Kittner H, Krugel U, El-Ashmawy IM et al (2000) Suppression of feeding-evoked dopamine release in the rat nucleus accumbens by the blockade of P2 purinoceptors. Eur J Pharmacol 406:R13–R14
Kittner H, Krugel U, Hoffmann E et al (2004) Modulation of feeding behaviour by blocking purinergic receptors in the rat nucleus accumbens: a combined microdialysis, electroencephalographic and behavioural study. Eur J Neurosci 19:396–404
Jin YH, Bailey TW, Li BY et al (2004) Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J Neurosci 24:4709–4717
Kato F, Shigetomi E (2001) Distinct modulation of evoked and spontaneous EPSCs by purinoceptors in the nucleus tractus solitarii of the rat. J Physiol 530:469–486
Khakh BS, Henderson G (1998) ATP receptor-mediated enhancement of fast excitatory neurotransmitter release in the brain. Mol Pharmacol 54:372–378
Shigetomi E, Kato F (2004) Action potential-independent release of glutamate by Ca2+ entry through presynaptic P2X receptors elicits postsynaptic firing in the brainstem autonomic network. J Neurosci 24:3125–3135
Khakh BS, Gittermann D, Cockayne DA et al (2003) ATP modulation of excitatory synapses onto interneurons. J Neurosci 23:7426–7437
Fellin T, Pozzan T, Carmignoto G (2006) Purinergic receptors mediate two distinct glutamate release pathways in hippocampal astrocytes. J Biol Chem 281:4274–4284
Inoue K, Nakazawa K, Fujimori K et al (1992) Extracellular adenosine 5′-triphosphate-evoked glutamate release in cultured hippocampal neurons. Neurosci Lett 134:215–218
Patti L, Raiteri L, Grilli M et al (2006) P2X7 receptors exert a permissive role on the activation of release-enhancing presynaptic alpha7 nicotinic receptors co-existing on rat neocortex glutamatergic terminals. Neuropharmacology 50:705–713
Papp L, Vizi ES, Sperlagh B (2004) Lack of ATP-evoked GABA and glutamate release in the hippocampus of P2X7 receptor -/- mice. Neuroreport 15:2387–2391
Lundy PM, Hamilton MG, Mi L et al (2002) Stimulation of Ca2+ influx through ATP receptors on rat brain synaptosomes: identification of functional P2X7 receptor subtypes. Br J Pharmacol 135:1616–1626
Miras-Portugal MT, Diaz-Hernandez M, Giraldez L et al (2003) P2X7 receptors in rat brain: presence in synaptic terminals and granule cells. Neurochem Res 28:1597–1605
Papp L, Vizi ES, Sperlagh B (2007) P2X7 receptor mediated phosphorylation of p38MAP kinase in the hippocampus. Biochem Biophys Res Commun 355:568–574
Armstrong JN, Brust TB, Lewis RG et al (2002) Activation of presynaptic P2X7-like receptors depresses mossy fiber-CA3 synaptic transmission through p38 mitogen-activated protein kinase. J Neurosci 22:5938–5945
Duan S, Anderson CM, Keung EC et al (2003) P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J Neurosci 23:1320–1328
Pannicke T, Fischer W, Biedermann B et al (2000) P2X7 receptors in Müller glial cells from the human retina. J Neurosci 20:5965–5972
Nakatsuka T, Tsuzuki K, Ling JX et al (2003) Distinct roles of P2X receptors in modulating glutamate release at different primary sensory synapses in rat spinal cord. J Neurophysiol 89:3243–3252
Nakatsuka T, Gu JG (2001) ATP P2X receptor-mediated enhancement of glutamate release and evoked EPSCs in dorsal horn neurons of the rat spinal cord. J Neurosci 21:6522–6531
Gu JG, MacDermott AB (1997) Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature 389:749–753
Li J, Perl ER (1995) ATP modulation of synaptic transmission in the spinal substantia gelatinosa. J Neurosci 15:3357–3365
Li P, Calejesan AA, Zhuo M (1998) ATP P2X receptors and sensory synaptic transmission between primary afferent fibers and spinal dorsal horn neurons in rats. J Neurophysiol 80:3356–3360
Krugel U, Schraft T, Regenthal R et al (2004) Purinergic modulation of extracellular glutamate levels in the nucleus accumbens in vivo. Int J Dev Neurosci 22:565–570
Price GD, Robertson SJ, Edwards FA (2003) Long-term potentiation of glutamatergic synaptic transmission induced by activation of presynaptic P2Y receptors in the rat medial habenula nucleus. Eur J Neurosci 17:844–850
Domercq M, Brambilla L, Pilati E et al (2006) P2Y1 receptor-evoked glutamate exocytosis from astrocytes: control by tumor necrosis factor-alpha and prostaglandins. J Biol Chem 281:30684–30696
Jeftinija SD, Jeftinija KV (1998) ATP stimulates release of excitatory amino acids from cultured Schwann cells. Neuroscience 82:927–934
Gomez-Villafuertes R, Gualix J, Miras-Portugal MT (2001) Single GABAergic synaptic terminals from rat midbrain exhibit functional P2X and dinucleotide receptors able to induce GABA secretion. J Neurochem 77:84–93
Hugel S, Schlichter R (2000) Presynaptic P2X receptors facilitate inhibitory GABAergic transmission between cultured rat spinal cord dorsal horn neurons. J Neurosci 20:2121–2130
Wirkner K, Kofalvi A, Fischer W et al (2005) Supersensitivity of P2X receptors in cerebrocortical cell cultures after in vitro ischemia. J Neurochem 95:1421–1437
Inoue K, Koizumi S, Ueno S et al (1999) The functions of ATP receptors in the synaptic transmission in the hippocampus. Prog Brain Res 120:193–206
Watano T, Calvert JA, Vial C et al (2004) P2X receptor subtype-specific modulation of excitatory and inhibitory synaptic inputs in the rat brainstem. J Physiol 558:745–757
Aihara H, Fujiwara S, Mizuta I et al (2002) Adenosine triphosphate accelerates recovery from hypoxic/hypoglycemic perturbation of guinea pig hippocampal neurotransmission via a P2 receptor. Brain Res 952:31–37
Wang CM, Chang YY, Kuo JS et al (2002) Activation of P2X7 receptors induced [3H]GABA release from the RBA-2 type-2 astrocyte cell line through a Cl-/HCO3 --dependent mechanism. Glia 37:8–18
Cunha RA, Ribeiro JA (2000) Purinergic modulation of [3H]GABA release from rat hippocampal nerve terminals. Neuropharmacology 39:1156–1167
Rhee JS, Wang ZM, Nabekura J et al (2000) ATP facilitates spontaneous glycinergic IPSC frequency at dissociated rat dorsal horn interneuron synapses. J Physiol 524(Pt 2):471–483
Wang ZM, Katsurabayashi S, Rhee JS et al (2001) Substance P abolishes the facilitatory effect of ATP on spontaneous glycine release in neurons of the trigeminal nucleus pars caudalis. J Neurosci 21:2983–2991
Kawamura M, Gachet C, Inoue K et al (2004) Direct excitation of inhibitory interneurons by extracellular ATP mediated by P2Y1 receptors in the hippocampal slice. J Neurosci 24:10835–10845
Vizi ES, Knoll J (1976) The inhibitory effect of adenosine and related nucleotides on the release of acetylcholine. Neuroscience 1:391–398
Silinsky EM, Ginsborg BL (1983) Inhibition of acetylcholine release from preganglionic frog nerves by ATP but not adenosine. Nature 305:327–328
Wiklund NP, Gustafsson LE, Lundin J (1985) Pre- and postjunctional modulation of cholinergic neuroeffector transmission by adenine nucleotides. Experiments with agonists and antagonists. Acta Physiol Scand 125:681–691
Wiklund NP, Gustafsson LE (1986) Neuromodulation by adenine nucleotides, as indicated by experiments with inhibitors of nucleotide inactivation. Acta Physiol Scand 126:217–223
Reese JH, Cooper JR (1982) Modulation of the release of acetylcholine from ileal synaptosomes by adenosine and adenosine 5′-triphosphate. J Pharmacol Exp Ther 223:612–616
Barajas-Lopez C, Muller MJ, Prieto-Gomez B et al (1995) ATP inhibits the synaptic release of acetylcholine in submucosal neurons. J Pharmacol Exp Ther 274:1238–1245
Giniatullin RA, Sokolova EM (1998) ATP and adenosine inhibit transmitter release at the frog neuromuscular junction through distinct presynaptic receptors. Br J Pharmacol 124:839–844
Smith AB, Hansen MA, Liu DM et al (2001) Pre- and postsynaptic actions of ATP on neurotransmission in rat submandibular ganglia. Neuroscience 107:283–291
Neal M, Cunningham J (1994) Modulation by endogenous ATP of the light-evoked release of ACh from retinal cholinergic neurones. Br J Pharmacol 113:1085–1087
De Lorenzo S, Veggetti M, Muchnik S et al (2006) Presynaptic inhibition of spontaneous acetylcholine release mediated by P2Y receptors at the mouse neuromuscular junction. Neuroscience 142:71–85
Stjarne L, Astrand P (1985) Relative pre- and postjunctional roles of noradrenaline and adenosine 5′-triphosphate as neurotransmitters of the sympathetic nerves of guinea-pig and mouse vas deferens. Neuroscience 14:929–946
Fujioka M, Cheung DW (1987) Autoregulation of neuromuscular transmission in the guinea-pig saphenous artery. Eur J Pharmacol 139:147–153
Shinozuka K, Bjur RA, Westfall DP (1988) Characterization of prejunctional purinoceptors on adrenergic nerves of the rat caudal artery. Naunyn Schmiedebergs Arch Pharmacol 338:221–227
Shinozuka K, Bjur RA, Westfall DP (1990) Effects of alpha,beta-methylene ATP on the prejunctional purinoceptors of the sympathetic nerves of the rat caudal artery. J Pharmacol Exp Ther 254:900–904
Forsyth KM, Bjur RA, Westfall DP (1991) Nucleotide modulation of norepinephrine release from sympathetic nerves in the rat vas deferens. J Pharmacol Exp Ther 256:821–826
von Kugelgen I, Kurz K, Starke K (1993) Axon terminal P2-purinoceptors in feedback control of sympathetic transmitter release. Neuroscience 56:263–267
Goncalves J, Queiroz G (1996) Purinoceptor modulation of noradrenaline release in rat tail artery: tonic modulation mediated by inhibitory P2Y- and facilitatory A2A-purinoceptors. Br J Pharmacol 117:156–160
von Kugelgen I, Kurz K, Starke K (1994) P2-purinoceptor-mediated autoinhibition of sympathetic transmitter release in mouse and rat vas deferens. Naunyn Schmiedebergs Arch Pharmacol 349:125–132
Kurz K, von Kugelgen I, Starke K (1993) Prejunctional modulation of noradrenaline release in mouse and rat vas deferens: contribution of P1- and P2-purinoceptors. Br J Pharmacol 110:1465–1472
von Kugelgen I, Stoffel D, Starke K (1995) P2-purinoceptor-mediated inhibition of noradrenaline release in rat atria. Br J Pharmacol 115:247–254
Fuder H, Muth U (1993) ATP and endogenous agonists inhibit evoked [3H]-noradrenaline release in rat iris via A1 and P2y-like purinoceptors. Naunyn Schmiedebergs Arch Pharmacol 348:352–357
Bohmann C, von Kugelgen I, Rump LC (1997) P2-receptor modulation of noradrenergic neurotransmission in rat kidney. Br J Pharmacol 121:1255–1262
Koch H, von Kugelgen I, Starke K (1998) P2-receptor-mediated inhibition of noradrenaline release in the rat pancreas. Naunyn Schmiedebergs Arch Pharmacol 357:431–440
Allgaier C, Wellmann H, Schobert A et al (1995) Cultured chick sympathetic neurons: modulation of electrically evoked noradrenaline release by P2-purinoceptors. Naunyn Schmiedebergs Arch Pharmacol 352:17–24
Allgaier C, Pullmann F, Schobert A et al (1994) P2 purinoceptors modulating noradrenaline release from sympathetic neurons in culture. Eur J Pharmacol 252:R7–R8
Lechner SG, Dorostkar MM, Mayer M et al (2004) Autoinhibition of transmitter release from PC12 cells and sympathetic neurons through a P2Y receptor-mediated inhibition of voltage-gated Ca2+ channels. Eur J Neurosci 20:2917–2928
Ennion SJ, Powell AD, Seward EP (2004) Identification of the P2Y12 receptor in nucleotide inhibition of exocytosis from bovine chromaffin cells. Mol Pharmacol 66:601–611
Trendelenburg AU, Cox SL, Gaiser EG et al (1999) Noradrenaline release from cultured mouse postganglionic sympathetic neurons: autoreceptor-mediated modulation. J Neurochem 73:1439–1445
Juranyi Z, Orso E, Janossy A et al (1997) ATP and [3H]noradrenaline release and the presence of ecto-Ca2+-ATPases in the capsule-glomerulosa fraction of the rat adrenal gland. J Endocrinol 153:105–114
O’Connor SC, Brain KL, Bennett MR (1999) Individual sympathetic varicosities possess different sensitivities to alpha 2 and P2 receptor agonists and antagonists in mouse vas deferens. Br J Pharmacol 128:1739–1753
Powell AD, Taschenmacher AG, Seward EP (2000) P2Y purinoceptors inhibit exocytosis in adrenal chromaffin cells via modulation of voltage-operated calcium channels. J Neurosci 20:606–616
von Kugelgen I, Spath L, Starke K (1994) Evidence for P2-purinoceptor-mediated inhibition of noradrenaline release in rat brain cortex. Br J Pharmacol 113:815–822
Koch H, von Kugelgen I, Starke K (1997) P2-receptor-mediated inhibition of noradrenaline release in the rat hippocampus. Naunyn Schmiedebergs Arch Pharmacol 355:707–715
von Kugelgen I, Koch H, Starke K (1997) P2-receptor-mediated inhibition of serotonin release in the rat brain cortex. Neuropharmacology 36:1221–1227
Trendelenburg AU, Bultmann R (2000) P2 receptor-mediated inhibition of dopamine release in rat neostriatum. Neuroscience 96:249–252
Bennett GC, Boarder MR (2000) The effect of nucleotides and adenosine on stimulus-evoked glutamate release from rat brain cortical slices. Br J Pharmacol 131:617–623
Mendoza-Fernandez V, Andrew RD, Barajas-Lopez C (2000) ATP inhibits glutamate synaptic release by acting at P2Y receptors in pyramidal neurons of hippocampal slices. J Pharmacol Exp Ther 293:172–179
Masino SA, Diao L, Illes P et al (2002) Modulation of hippocampal glutamatergic transmission by ATP is dependent on adenosine A1 receptors. J Pharmacol Exp Ther 303:356–363
Borvendeg SJ, Gerevich Z, Gillen C et al (2003) P2Y receptor-mediated inhibition of voltage-dependent Ca2+ channels in rat dorsal root ganglion neurons. Synapse 47:159–161
Illes P, Gerevich Z (2004) P2Y receptor and pain transmission. Purinergic Signalling 1:3–10
Gerevich Z, Borvendeg SJ, Schroder W et al (2004) Inhibition of N-type voltage-activated calcium channels in rat dorsal root ganglion neurons by P2Y receptors is a possible mechanism of ADP-induced analgesia. J Neurosci 24:797–807
Koizumi S, Fujishita K, Tsuda M et al (2003) Dynamic inhibition of excitatory synaptic transmission by astrocyte-derived ATP in hippocampal cultures. Proc Natl Acad Sci U S A 100:11023–11028
Bowser DN, Khakh BS (2004) ATP excites interneurons and astrocytes to increase synaptic inhibition in neuronal networks. J Neurosci 24:8606–8620
Sperlágh B, Zsilla G, Baranyi M et al Purinergic modulation of glutamate release under ischemic-like conditions in the hippocampus. Neuroscience (in press)
Acknowledgments
This study was supported by a grant from the Hungarian Research and Development Fund (NKFP1A/002/2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Sperlágh, B., Heinrich, A. & Csölle, C. P2 receptor-mediated modulation of neurotransmitter release—an update. Purinergic Signalling 3, 269–284 (2007). https://doi.org/10.1007/s11302-007-9080-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-007-9080-0