Abstract
Metabotropic pyrimidine and purine nucleotide receptors (P2Y receptors) belong to the superfamily of G protein-coupled receptors (GPCR). They are distinguishable from adenosine receptors (P1) as they bind adenine and/or uracil nucleotide triphosphates or diphosphates depending on the subtype. Over the past decade, P2Y receptors have been cloned from a variety of tissues and species, and as many as eight functional subtypes have been characterized. Most recently, several members of the P2Y12-like receptor group, which includes the clopidogrel-sensitive ADP receptor P2Y12, have been deorphanized. The P2Y12-like receptor group comprises several structurally related GPCR which, however, display heterogeneous agonist specificity including nucleotides, their derivatives, and lipids. Besides the established function of P2Y12 in platelet activation, expression in macrophages, neuronal and glial cells as well as recent results from functional studies implicate that several members of this group may have specific functions in neurotransmission, inflammation, chemotaxis, and response to tissue injury. This review focuses specifically on the structure-function relation and shortly summarizes some aspects of the physiological relevance of P2Y12-like receptor members.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most cellular effects of nucleotides are mediated through two main families of specific cell surface receptors, the ionotropic P2X and metabotropic P2Y nucleotide receptors. The metabotropic receptors belong to the superfamily of G protein-coupled receptors (GPCR). The GPCR superfamily comprises at least five structurally distinct families/subfamilies (GRAFS classification) which share little sequence homology among each other [1]. The rhodopsin-like receptors (also called family A or 1) form the largest family in vertebrates. Eight different mammalian P2Y (P2Y1, 2, 4, 6, 11–14) receptor subtypes, all belonging to the rhodopsin-like receptors (family A), have been identified and proven to function as receptors for extracellular nucleotides so far [2]. Based on their preferential agonists, P2Y receptors have been subclassified into adenine nucleotide-activated (P2Y1, P2Y11, P2Y12, P2Y13), pyrimidine nucleotide-activated (P2Y4, P2Y6), ATP/UTP-activated (P2Y2), and UDP-sugar-activated (P2Y14) receptors. Most structural and functional studies were performed with the classic P2Y receptors P2Y1 and P2Y2 [3]. However, in comparison to other GPCR, large-scale structure/function data are rare, mainly due to difficulties in finding suitable P2Y-free heterologous expression systems [4]. Pharmacological characterization on both native and recombinant P2Y receptors is further hampered by the release of endogenous nucleotides and the hydrolysis or conversion of exogenous and endogenous nucleotides by ectoenzymes (for review see [5]). ATP and AMP are metabolized by cell surface enzymes, the ecto-NTPDase 1 (nucleoside triphosphate diphosphohydrolase 1, ectoapyrase, CD39, metabolizes ATP to AMP) and the 5′-ectonucleotidase (CD73, metabolizes AMP to adenosine). In addition, a nucleoside diphosphokinase catalyzes the transfer of the gamma phosphate of nucleoside triphosphates to nucleoside diphosphates. For example, in the presence of ATP, nucleoside diphosphokinase catalyzes the conversion of UDP to UTP. These specific problems may have contributed to many false and controversial declarations of P2Y receptors being activated by nucleotides [6–9]. Recent success in expressing GPCR in yeast, which is discussed below, may circumvent these problems and may engage structure-function-relation studies.
Although in-depth phylogenetic analysis of GPCR groups indicates clustering of P2Y receptors, when compared with other GPCR of family A [1, 10], the adenine nucleotide-activated P2Y1 and P2Y12, for example, display only minor structural relation at the protein level. Phylogenetic analysis even revealed that nucleotide specificity e.g. for ADP evolved independently in P2Y1 and P2Y12. So we suggest that grouping of P2Y receptors should be performed on phylogenetic aspects rather than on ligand specificity.
Based on their amino acid sequences P2Y receptors can be subdivided into at least two groups (Fig. 1). One comprises P2Y1, 2, 4, 6, 11, and the second group contains P2Y12–14. Additionally, several other structurally related GPCR, such as CysLT1R, GPR91, GPR99, and GPR34, cluster into both groups (Fig. 1). These receptors are activated by structurally different ligands such as leukotrienes, organic acids, and phospholipids. Probably because of their structural relation several P2Y receptors display agonist promiscuity. It was shown that not only ADP but also leukotriene E4 (LTE4) activates P2Y12 at nanomolar concentrations [11]. Cysteinyl leukotriene receptors (CysLT1R and CysLT2R) have been described to be activated by UDP in addition to activation by cysteinyl leukotrienes [12, 13]. Further, there is evidence that CysLT1R antagonists, such as pranlukast and montelukast, inhibit activation of P2Y1 and P2Y6 by 2MeS-ADP (2-methylthio-ADP) and UDP, respectively [14]. Although P2Y12 and cysteinyl leukotriene receptors are clinically used targets for prevention of thrombocyte aggregation (clopidogrel) and treatment of asthma (montelukast), respectively, the structural basis and (patho)physiological relevance of dual/multiple agonist specificity is obscure. Close structural relations between P2Y and other non-nucleotide receptors as well as the mentioned examples of ligand promiscuity indicate that nucleotides are probably only one of many natural agonists on the genuine P2Y receptors.
Phylogenetic relation of human P2Y receptors. To evaluate the structural relation of P2Y receptors and related GPCR, amino acid sequences of human orthologs were aligned and phylogenetic relations were estimated using CLUSTAL W. The derived tree was essentially identical to former analysis [2, 10]. The P2Y12-like receptors cluster into a group (framed) that is distinct from other nucleotide receptors such as P2Y1 and P2Y2
Structural evolution of P2Y12-like GPCR
Discovery and deorphanization of group members
The ADP-(P2Y12)-like receptor group is the most recently identified P2Y receptor group and includes P2Y12, P2Y13, and P2Y14 (see Fig. 1, Table 1). Based on high structural similarity, the lyso-phosphatidylserine receptor (GPR34) and several orphan GPCR (GPR34-like, GPR82, GPR87, GPR171) are considered members of the structural P2Y12-like receptor group (3,11). Most members of this group were deorphanized by the so-called reverse pharmacology approach. The orphan receptors predicted from sequence data are now often used to ascertain the ligands by testing them on tissue extracts and subsequent fractionation or on huge libraries of bioactive compounds, whereas, traditionally, the bioactive ligand was used to identify the receptor (classic approach).
As the first member of the P2Y12-like receptor group P2Y14 (synonyms KIAA0001, GPR105, VTR 15–20) was identified [15, 16] and then deorphanized in the year 2000 [17]. UDP-glucose was found to activate P2Y14 by screening multiple receptors, each expressed in individual yeast strains, against a large library of over 700 known and putative natural GPCR agonists.
In 2001, P2Y12 (initially named SP1999) was discovered and identified as the platelet clopidogrel-sensitive ADP receptor by a number of groups [18–21]. The P2Y12 was deorphanized with both the classic and the reverse pharmacology approaches. Hollopeter et al. used subfractionated transcripts from platelets and isolated the cRNA (complementary RNA, encoding e.g. the P2Y12 receptor) which mediated ADP-induced increases in K+ current after injection into Xenopus oocytes [19]. In contrast, fractionated rat spinal cord extracts were assayed for Ca2+ mobilization in cells transiently transfected with P2Y12 and chimeric Gα subunit [21]. Almost in parallel, ADP was shown to be an agonist for P2Y13 (former GPR86), a very close structural neighbor of P2Y12 [22–24]. 2MeS-ADP is the most potent agonist at P2Y12 followed by ADP, adenosine 5′-O-2-thiodiphosphate, and ATP. (N)-methanocarba-2-methylthio-ADP, a highly potent agonist at P2Y1, exhibited no and only minor agonist activity at P2Y12 and P2Y13, respectively [25].
Based on structural relations GPR34 [26, 27], GPR82 [28], GPR87 [29], and GPR171 (synonym H963) [30] also belong to the P2Y12-like group (see Fig. 1). Except for GPR34, which was recently identified as a receptor for lyso-phosphatidylserine [31] by a reverse pharmacology approach, all the other P2Y12-like receptors are still orphan GPCR.
The evolutionary origins
Based on structural similarities P2Y12-like receptors cluster into a group distinct from other P2Y receptors (see Figs. 1 and 2). This P2Y12-like receptor group can be subdivided into two subgroups. One subgroup encompasses P2Y12, P2Y13, P2Y14, GPR87, and GPR171, the other subgroup GPR34, GPR34-like, and GPR82 (Fig. 2). This may suggest that both subgroups evolved from gene duplications starting from two related members. The grouping is further supported by shared genomic localization. Chromosomal clustering in the human genome is found for P2Y12, P2Y13, P2Y14, GPR87, and GPR171 at 3q24-3q25 as well as for GPR34 and GPR82 at Xp11.4. This clustering is evolutionary well preserved. In the zebrafish genome P2Y12, P2Y14/GPR87, and GPR171 cluster at chromosome 15 and GPR34 type 2 and GPR82 are tandemly arranged at chromosome 9. Gene clustering is not only found for vertebrate P2Y12-like receptors but also for several other GPCR genes. Multiple copies of related GPCR genes, such as the human protease-activated receptors on 5q13, trace amine-associated receptors (TAAR, human 6q23.2), and CC-chemokine receptors (human 3p21.3), are the result of intrachromosomal gene duplications. The most impressive example is found in odorant receptors, where in humans, chromosome 11 contains nearly half of the odorant receptor repertoire, including a single cluster of more than 100 odorant receptors [32]. Gene amplification can be viewed as a dynamic and reversible regulatory mechanism that facilitates adaptation to variable environments. Clustered genes may confer selective benefits via their ability to be co-regulated and co-amplified. Indeed, even transcripts encoding a fusion protein of GPR34 and GPR82 have been observed (own unpublished results). However, the biological relevance of P2Y12-like receptor clustering in respect to transcriptional activity at their respective genomic loci, co-regulation, and transcript diversity need to be determined in the future.
Evolutionary origin of P2Y12-like receptors. To subclassify and evaluate the phylogenetic relations between the P2Y12-like receptors, the amino acid sequences of orthologs from one species of each vertebrate class (if available) were aligned using CLUSTAL W (identity matrices) and a phylogenetic tree was constructed (1,000 iterations). Bootstrap values >600 were considered as significant to support a new branch
Since P2Y12, P2Y14/GPR87, GPR34, and GPR34-like are all found in sharks (Mustelus manazo, Carcharodon carcharias) and chimeras (Callorhinchus milii) one can assume that P2Y12-like receptors arose more than 450 million years (Myr) ago, before cartilaginous and bony fish split. Our own polymerase chain reaction (PCR) amplification attempts as well as the ongoing lamprey (Petromyzon marina) and sea urchin (Strongylocentrotus purpuratus) genome projects revealed no P2Y12-like sequences yet, suggesting their origin in the very early Gnathostomata or, less likely, a loss in agnate and all non-vertebrates.
Gene duplication can be a primary source of the genetic material from which genes with new functions evolve. One copy of a duplicated gene may become mutated and acquire unique functionality without risking the fitness of the organism ensured by the homolog. On the other hand, if not advantageous, continuous accumulation of mutations (neutral drift) will eliminate one of the genes, a process named pseudogenization. In fish genomes, there is only one respective ortholog with relation to P2Y12/P2Y13 and to GPR87/P2Y14. In the case of P2Y12/P2Y13 the fish ortholog is more closely related to P2Y12 implicating that P2Y13 evolved later in tetrapod evolution probably by P2Y12 duplication. However, one cannot rule out that the gene was eliminated in the common ancestor of sharks and bony fishes. The evolutionary mechanism of P2Y14 and GPR87 evolvement remains unsolved since their structural relation to the fish orthologs is similar. The absence of P2Y14 in the African craw frog (Xenopus) genome but the presence of ortholog sequences in all other sequenced tetrapod genomes does not necessarily implicate that P2Y14 derived from GPR87 in early tetrapod evolution. Again, P2Y14 gene loss in amphibians must be considered.
Orthologs of GPR34, GPR87/P2Y14, and P2Y12 are found in all tetrapods and bony and cartilaginous fishes investigated so far. However, not all members of the P2Y12-like group appear to be present in all vertebrate classes. GPR82 has an ortholog in zebrafish but is not yet found in the genomes of pufferfishes and the lizard (Anolis carolinensis) genome. Although GPR171 is found in reptiles, birds, mammals, zebrafish, and chimera it appears to be absent in African craw frog, stickleback, and pufferfishes. This may implicate specific functions of some P2Y12-like receptors which are less important in some species or are compensated by other receptors and mechanisms.
Signatures of gene inactivation (pseudogenization) are rarely found for members of the P2Y12-like group. Following gene duplication in the common ancestor of evolutionary basic fishes, like eels and carps [33], pseudogenization of one GPR34 subtype was identified in a salmon species (Keta salmon) (own unpublished results). The GPR34-like receptor is an evolutionary old P2Y12-like receptor being present in cartilaginous and bony fishes, amphibians, and birds but not in mammals (Fig. 2). Sequences with residual relation to the GPR34-like receptor are found in the platypus genome but it is most likely a pseudogene.
Genomic organization of P2Y12-like receptors
Comparison of transcript and genomic sequences provides information on intron/exon structure of a GPCR gene. Since the gain or loss of spliceosomal introns are unique events in evolution, they can serve as markers for phylogenetic analysis. Further, such analyses may reveal splice variants and may be informative about the promoter structure and gene regulation. Introns are the basis of alternative splicing, exon skipping, and RNA editing events and, therefore, can contribute to receptor diversity at a supragenomic level.
Most coding regions of human P2Y12-like receptors do not contain introns (Table 2). One exception is GPR87 where the genomic sequence encoding the receptor’s N terminus is interrupted by an intron in mammalian and avian orthologs. Another intron which is rarely removed (cryptic intron) has been identified in the N terminus-encoding sequence of GPR34 [34].
The genomic organization of the individual P2Y12-like receptor genes is not well conserved during vertebrate evolution (see Table 2). For example, the P2Y12 gene gained an intron in bony fish evolution disrupting the open reading frame of the transmembrane domain 2 (TMD2)-encoding part. Similarly, one of the two GPR34 paralog genes acquired an intron in the more recent bony fish evolution [33]. The two long-standing alternative explanations for the origin of introns, the intron-early theory and intron-late theory, remain a matter of continuous debate not only for GPCR [35]. The intron-early theory suggests that introns are extremely ancient characteristics of genes and that early genes were created through the intron-mediated shuffling of exons. However, numerous gene and genome comparison studies provided evidence that at least some introns are more recently acquired (intron-late theory). The P2Y12-like receptor group is, therefore, a nice example where introns were acquired in the coding region in some species during more recent evolution.
In contrast to the coding region, the 5′ region of most members of the P2Y12-like receptor group displays a distinct intron/exon organization with sometimes multiple transcription starts. Also, the gene sizes strongly differ between the individual and even closely related receptors. Introns in the 3′-untranslated region (UTR) of P2Y12-like receptors have not been found yet. Large and complex organized 5′ non-coding regions of a gene may provide the basis for multiple promoter regions, cis-acting elements, and a variable 5′ UTR of the mRNA. Alternative structures of the 5′ UTR can contribute to expression regulation and alternative translation start points. For example, 5′ UTRs often contain small open reading frames (ORF) which can be translated via leaky scanning at the ribosome. Such leaky scanning can reduce translation of the downstream main ORF as shown for several genes including GPCR [36, 37].
It is of interest to note that both genes, GPR34 and GPR82, are located in antisense orientation within a large intron of the CASK gene. This position is conserved during vertebrate evolution. The CASK gene encodes a calcium/calmodulin-dependent serine protein kinase that is a member of the membrane-associated guanylate kinase (MAGUK) protein family. Since GPR34 and GPR82 transcripts are antisense orientated to CASK one can speculate that transcripts may regulate expression of CASK or vice versa. Such hypotheses will be addressed in receptor-deficient mouse models (see below) in the future.
Key residues defining the individual member
Many attempts have been made to identify structural signatures which are helpful in annotation and grouping of P2Y receptors. On the basis of available sequence data for validated P2Y receptors, key residues were extracted to define P2Y12-like and P2Y1-like receptor groups [11, 38, 39]. In recent studies, we have shown for several GPCR that a significant number of orthologs is required for identification of functional motifs and key residues [33, 40–42]. By mining public databases and by amplifying and sequencing P2Y12-like orthologs we have acquired large sets of sequences of P2Y12 (74 orthologs), P2Y13 (31 orthologs), P2Y14 (38 orthologs), GPR87 (51 orthologs), GPR171 (41 orthologs), GPR82 (34 orthologs), and GPR34 (133 orthologs) to determine structural conservation of the members and to identify amino acid sequence motifs and key residues that are unique for the P2Y12-like receptor group and the individual members (Fig. 3). These data sets were obtained from vertebrate species ranging from evolutionary old bony and cartilaginous fishes to the more modern mammals representing 450 Myr of evolution. The overall identity between P2Y12-like members is rather low ranging from 19% amino acid identity (human GPR82 vs human GPR87) to 47% (human P2Y12 and human P2Y13). Between the respective fish and mammal receptor orthologs P2Y12 (~49%) shows the highest identity followed by GPR171 (~48%), GPR34 (~40%), and GPR82 (~35%). Since not all P2Y12-like GPCR have orthologs in all vertebrate classes, we analyzed the structural conservation within this receptor group by comparing K a/K s values (ratio of the number of nonsynonymous substitutions per nonsynonymous site and the number of synonymous—or silent—substitutions per synonymous—or silent—site) of the P2Y12-like receptor ortholog set from species containing all group members (Table 3). Although all P2Y12-like receptors display a purifying selection mode of evolution (K a/K s <<1 indicates high conservation and elimination of deleterious mutations), there are significant differences between the members of this receptor group. P2Y12 and GPR87 were kept most conserved during evolution in birds and mammals. By contrast, GPR82 appears to be less constrained as already indicated by a relatively low conservation at the amino acid level (see above).
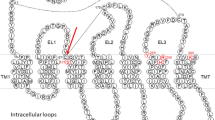
Conserved residues in P2Y12-like receptors. To identify conserved group and member-specific positions the amino acid sequences of P2Y12 (74 orthologs), P2Y13 (31 orthologs), P2Y14 (38 orthologs), GPR87 (51 orthologs), GPR171 (41 orthologs), GPR82 (34 orthologs), and GPR34 (133 orthologs) were aligned using CLUSTAL W. Residues that are 100% conserved among the respective orthologs were boxed. Only a few positions are almost fully conserved among all members of the P2Y12-like group (position number refers to the relative numbering system by Ballesteros and Weinstein [73]): TMD1: Phe/Tyr1.39, Phe1.57; TMD2: Leu2.43, Asn/Asp2.50, Pro/Ala2.58; TMD3: Tyr/His3.33, Arg/Gln3.50; TMD4: Trp4.50; TMD6: Cys/Ser6.47; Pro6.50; TMD7: Asp7.49, Pro7.50, and the two Cys residues bridging extracellular loops 1 and 2. The approximate positions of the seven transmembrane domains (TMD) are given above the sequences
As shown in Fig. 3, members of the P2Y12-like group share only eight fully conserved residues (Phe1.57, Leu2.43, Trp4.50, Pro6.50, Asp7.49, Pro7.50, and both Cys residues bridging extracellular loops 1 and 2) when sequences of more than 400 receptors of this group are compared. Although the ADP receptors P2Y12 and P2Y13 share more than 50 fully conserved residues none of these conserved residues is exclusively found in P2Y12 and P2Y13 but rather present also in other P2Y12-like receptors (see Fig. 3). Further, only a few residues are member specifically conserved (GPR171: Gln3.53 and Asn5.59; P2Y13: Met7.48; GPR82: Leu6.44 and Asp7.57; GPR34: Met7.52) and have not been found in other P2Y12-like receptors so far. These facts suggest that ligand and signaling specificity is determined by a combination of many, more or less conserved determinants. It has been proposed that His6.52/Arg6.55 and Lys7.35/Glu7.36/Leu7.39 within TMD6 and the ECL3, respectively, may present such motifs required for nucleotide binding [38]. However, such residue combination is also present in some GPR87, GPR171, and GPR34 orthologs which are not activated by ADP. This does not rule out that these residues are involved in nucleotide binding of e.g. P2Y12 but it implicates additional positions which determine ligand specificity. In-depth structure-function analysis e.g. by mutagenesis studies are required to identify key positions and their structural properties.
Physiological relevance of P2Y12-like receptors
Functional characterization of P2Y12-like receptors in heterologous expression systems
Signal transduction of the ADP receptor P2Y12 via pertussis toxin-sensitive Gi proteins and adenylyl cyclase inhibition is well established [22]. Similar Gi protein-coupling specificity was found for P2Y13 [22, 24], P2Y14 [43], and lyso-PS receptor GPR34 [31, 33]. Because adenylyl cyclase inhibition assays are usually less sensitive and robust several other experimental setups were established to measure function of P2Y12-like GPCR. It has been shown that Gα15 and Gα16 can be activated by a wide variety of GPCR [44]. The ability of Gα16 to bypass the selectivity of receptor/G protein interaction was also useful to measure activation of P2Y13 [45]. It has been demonstrated that replacement of the four or five C-terminal amino acids of Gαq with the corresponding Gαi residues (referred to as GΔ6qi4 [46]) confers the ability to stimulate the PLC-β pathway onto Gi-coupled receptors [47]. Successful heterologous expression and activation by applying chimeric Gαqi4 has been demonstrated for P2Y12–14 and lyso-PS receptor GPR34 [25, 33, 48].
We have previously shown that P2Y12 as well as the lyso-PS receptor GPR34 display increased basal activity in functional assays when compared with other GPCR [33]. This high basal activity can be either discussed as the natural ground state of the receptor activity equilibrium or as the effect of receptor agonists that are present in the cell culture medium or that are released from cells. Analyzing mutations in the highly conserved DRY motif of P2Y12 we recently showed that basal activity is abolished in the DHY mutant but agonist-induced activation remains intact (Fig. 4). We conclude that the basal activity of the ADP receptor is rather a genuine property of this receptor and not due to continuous stimulation by agonists. High basal activity appears to be a general feature of all members of the P2Y12-like receptor group. In our initial ortholog screen we identified two GPR87/P2Y14 receptors, carp GPR87/P2Y14 types 1a and 1b (AY241103, AY241102), which differ in only ten amino acid positions. Interestingly, carp GPR87/P2Y14 type 1b displays higher basal activity and studies are ongoing to identify residues that promote constitutive activity.
High basal activity is a genuine property of P2Y12. Basal activity of the wild-type P2Y12 (DRY motif) and a mutant P2Y12 (DHR motif) was determined in transiently transfected COS-7 cells. Gi coupling of P2Y12 was rerouted to IP production by co-transfection of a chimeric G protein GΔ6qi4 [46]
Because many standard mammalian expression systems endogenously express P2Y receptors clear-cut functional studies are difficult to perform. One exception is the 1321N1 human astrocytoma cell line which does not express P2Y receptors. However, transient expression of GPCR in 1321N1 cells is limited by low transfection efficiency and stable transfection of these cells is usually required. Heterologous expression of GPCR in yeast was initially established for large-scale purification of receptor proteins. These advances in the expression of heterologous GPCR in the yeast Saccharomyces cerevisiae have led to the development of sensitive and selective assays of ligand-induced GPCR activation (reviewed in [49]). To facilitate a more systematic genetic analysis of GPCR function, e.g., by saturating random mutagenesis, we took advantage of a yeast expression system in which parts of the mammalian GPCR signaling system (GPCR and chimeric G protein) are linked to a modified yeast pheromone pathway (Fig. 5) [50]. The coupling of receptor activity to the genetically engineered yeast pathway allows for rapid and economical screening of substance libraries and randomly modified receptor libraries. Functional studies in yeast may have an advantage especially for P2Y receptors because one can (at least partially) circumvent specific problems in working with nucleotide receptors (see above). Only two NTPDases (GDA1 and YND1/APY1) have been found within the entire yeast genome which are mainly expressed in the Golgi apparatus [51]. Although conversion of nucleotides by nucleotidases and ectoapyrases occur also in yeast and one cannot exclude conversion of extracellular nucleotides by yeast enzymes, there is no endogenous plasma membrane P2Y receptor in yeast which can mediate and, therefore, interfere with transmembrane signaling of heterologously expressed P2Y receptors in yeast. We and others have successfully used this system for expression and functional study of P2Y12 [52], P2Y14 [53], and lyso-PS receptor GPR34 (unpublished data). As shown in Fig. 6a, P2Y12-expressing yeast cells grow only in the presence of the agonist 2-methylthioadenosine 5′-diphosphate (2MeS-ADP).
Functional expression and in vitro evolution of GPCR in yeast. Genetically modified yeast cells are transformed with a mammalian GPCR. Following agonist activation and constitutive receptor activity, the receptor couples to a chimeric G protein [backbone yeast G protein Gpa1 in which the C-terminal five amino acids were replaced by the respective mammalian G protein sequence (e.g., from the Gi protein)]. Activation of the chimeric G protein enables yeast cells to grow on histidine-free medium by utilizing parts of the yeast mating pathway [49]. Gpa yeast ortholog of the mammalian G protein alpha subunit, Ste4 yeast ortholog of the mammalian G protein beta subunit, Ste18 yeast ortholog of the mammalian G protein gamma subunit, Ste12 transcription factor that is activated by a MAP kinase signaling cascade, Far1 cell cycle regulator that directly inhibits the yeast cyclin-dependent kinase Cdc28-Cln
Functional expression and random mutagenesis of P2Y12 in yeast. a The human P2Y12 receptor was transformed into modified yeast (see Fig. 4). The agonist 2-methylthioadenosine 5′-diphosphate (2MeS-ADP) induces a robust yeast cell growth. b The entire coding region of the human P2Y12 was subjected to random mutagenesis and transformed yeast cells were selected for growth in agonist-free U−/H−medium. Colonies that grow under this condition contain a constitutively active P2Y12. Exemplarily, mutations of a triple mutant (L115Q/F177S/R224G) were individualized and tested separately for constitutive activity. The data indicate that L115Q mainly contributes to the constitutive activity of the triple mutant
Constitutively active GPCR are useful tools in studying the action of inverse agonists and activation mechanisms in GPCR. A constitutively active P2Y12 was generated by replacing the endogenous C terminus with the corresponding part of the human P2Y1 receptor [54]. Pharmacological evaluation of several P2Y12 antagonists revealed AR-C78511 (an adenosine derivative) as a potent P2Y12 inverse agonist. Traditional mutagenesis approaches are limited to screen for activating mutations because the number of mutant proteins that can be investigated is usually relatively small, primarily because of technical reasons and the time and costs needed to generate and analyze large numbers of mutant receptors. To circumvent these limitations, yeast has emerged as a highly useful host for the in vivo reconstitution of mammalian GPCR. Therefore, we applied a random mutagenesis and screening approach in yeast to identify key residues in maintaining ground stage of the human P2Y12. PCR-based random mutagenesis was optimized to induce approximately four mutations in 1 kbp. Several dozen clones were selected and are now under in-depth investigation. For example, we identified a triple mutant P2Y12 (Leu115Gln/Phe177Ser/Arg224Gly), and individual characterization revealed Leu115Gln (TMD3) to be mainly responsible for constitutive activity in the yeast expression system (Fig. 6b). These promising results in applying the yeast expression system await further efforts to identify functionally relevant determinants in P2Y12-like receptors.
Establishing in vivo function of P2Y12-like receptors using mouse models
Selective receptor ligand and receptor-deficient animal models are suitable tools to evaluate the physiological relevance of distinct GPCR. Enormous efforts have been undertaken in the development of selective and clinically useful ADP and other nucleotide receptor ligands. But except for P2Y12, dissection of the physiological relevance of all other P2Y12-like GPCR is at the very early stage. Since the pharmacological properties of ligands have been reviewed in detail elsewhere [2] we only shortly summarize available data on receptor-deficient animal models.
Based on the clinical success of irreversibly bound P2Y12 antagonists, such as clopidogrel, the pivotal role of ADP in arterial thrombogenesis is well established. Although the combined action of P2Y1 and P2Y12 is necessary for the full platelet aggregation response to ADP, mice deficient for P2Y12 already display reduced platelet adhesion/activation, thrombus growth, and stability [18, 55]. Except for the altered platelet functionality P2Y12-deficient mice appear normal under standard laboratory conditions. In consent with this finding, we occasionally identified a frameshift (T insertion at base pair position 667) in the P2Y12 coding sequence of several individuals of the Asian house mouse (inbred laboratory strain of Mus musculus castaneus from of the Laboratoire Génome Populations Interactions Adaptation at the Universite Montpellier, France) during ortholog screening which showed no obvious phenotype in standard laboratory captivity. The platelet function was, however, not tested. The identified P2Y12 inactivation in some Asian house mice may reflect either natural polymorphisms present in the wild population or a new polymorphism that has been introduced during captivity. To distinguish between these possibilities, we analyzed the position 667 in P2Y12 from 88 wild M. m. castaneus trapped in Taiwan [41]. All contained the intact P2Y12 allele indicating that the inactive P2Y12 allele is very rare or absent in this wild M. m. castaneus population and favors the hypothesis of its acquisition in captivity (unpublished observations).
The fact that receptor deficiency is per se compatible with viability and fertility appears to be true also for other P2Y12-like receptor-deficient species and mouse models. For example, mice individually lacking GPR82 and GPR34 are viable, fertile, and produced viable offspring (own unpublished observation). Further, there are several vertebrate classes and species naturally deficient in distinct P2Y12-like receptors. For example, P2Y13 is absent in fish genomes sequenced so far, GPR82 is not present in pufferfish genomes, and GPR171 is absent in pufferfish and Xenopus genomes.
It appears that many functions of P2Y12-like receptors are more distinct and their disclosure requires specific challenging conditions. Indeed, P2Y12-deficient mice revealed an unexpected phenotype when specifically challenged. CNS injury is accompanied by release of nucleotides, serving as signals for microglial activation or chemotaxis. Microglia cells express several purinoceptors, including P2Y12. Microglia in P2Y12-deficient mice showed significantly diminished directional branch extension toward sites of cortical damage in the living mouse. These results imply that P2Y12 is a primary site at which nucleotides act to induce microglial chemotaxis at early stages of the response to local CNS injury [56].
Variants of P2Y12-like receptor genes within human populations
Activating and inactivating mutations in GPCR have been made responsible for more than 30 different human diseases [57]. As expected from its pivotal role in platelet activation inactivating mutations in P2Y12 can cause a congenital bleeding disorder. Only a few missense (Met1Arg, Pro258Thr, Arg256Gln, Arg265Trp) and frameshifting (frameshift at amino acid position 240) mutations have been reported [19, 58–60]. Unexceptionally, the missense mutations found are at highly conserved positions and cause an impairment of receptor function. There are no other human diseases identified yet which are associated with dysfunction of P2Y12-like receptors.
The antithrombotic effect of clopidogrel is considerably variable and the P2Y12 gene was screened for possible sequence variants. Five nucleotide variations were found in the human P2Y12 gene, two of them silent substitutions in the coding region [61]. Several studies were initiated to investigate the impact of P2Y12 polymorphisms on atherosclerosis [62, 63] and clopidogrel efficiency in preventing neurological events [64] showing no or some association to one haplotype. In single nucleotide polymorphism (SNP) projects, such as HapMap [65], SeattleSNP etc., silent variations have been identified at amino acid position Val4, Asn6, Gly12, and Phe182 of P2Y12. For example the C/T variation at amino acid position Phe182 is only found in the African population whereas the G/T variation at position Gly12 is present only in European and Asian populations. One missense mutation (Glu330Gly; receptor C terminus) has been detected in SNP projects. This variant is absent in European and Asian populations but displays an allelic frequency of about 15% in the African population. The functional relevance of this missense mutation has not been studied yet but a Gly at position 330 is naturally found in many other P2Y12 orthologs including primate P2Y12 almost ruling out a specific input in receptor function.
One silent variant (C/T at Ile59) and one substituting variant (T/C at Met158Thr) were identified in the coding region of P2Y13. The Thr158 variant is less frequent in African populations (~4%) when compared to European (~21%) and Asian (~18%) populations. Met158 is not conserved and a Thr at this position is found in the dolphin (Tursiops truncates).
Only two silent substitutions (A/G at Ala35 and T/C at Phe240) have been identified in P2Y14 so far. The GPR171 gene contains two more frequent silent substitutions (T/C at Tyr19, A/C Thr58) and one missense variant (A/G at Ile283Val, frequency about 6%) which is located within the DPXXY motif of TMD7. Ile283 is quite conserved among vertebrate GPR171 orthologs. The functional relevance of Val283 has not been studied yet but it occurs naturally in the zebrafish ortholog.
In the human GPR87 gene three silent polymorphisms (G/A at Pro46, G/A at Leu179, C/T at Tyr355) and one nonsynonymous polymorphism (C/T at Thr205Met) are present. The position 205 is not conserved and Met205 is found in several rodents. Only rare silent substitutions in the coding regions have been found in GPR82 (C/T at Asp313) and in GPR34 (A/G at Val296) [34].
SNP stochastically occur in individual genomes and can amount to a reasonable frequency in populations by drift but also by selection. There are several approaches and methods which are suitable to distinguish between drift and selection. Population genetic models predict that selection can leave “footprints” in closely linked genomic regions. Several methods were developed to detect signatures of selective sweeps in genomic sequences [66]. All methods require data on allele variation (mainly SNP) within populations. Large genome-wide analyses have scanned the human genome for signatures of positive selection on the basis of nonsynonymous and synonymous substitution ratios or single nucleotide polymorphism (SNP) data. Several loci which contain GPCR genes have been identified using such methods, but the P2Y12-like receptor-containing loci showed no strong signatures of recent positive selection in these studies [67–70].
Conclusion
P2Y12-like receptors which are grouped mainly by phylogenetic relations have been an inherent part of the vertebrate GPCR repertoire since more than 450 Myr. Although sharing features in respect to structural determinants and signal transduction the activating ligands are heterogeneous such as nucleotides, nucleotide derivatives, leukotrienes, and phospholipids. We are at the very beginning of understanding the physiological importance of the individual members. The nature of the ligands, first functional data, and expression of several members in migrating cells point at functions in immunologic response and tissue damage response. Doubtless, upcoming receptor-deficient mouse models and selective receptor ligands will help to unveil the functions of P2Y12-like receptor members. Further, one should consider combined receptor-deficient mouse models within this group but also with other P2Y receptors to uncover phenotypes which are hidden by receptor redundancy, e.g., in the case of ADP receptors.
References
Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB (2003) The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol 63(6):1256–1272
von Kugelgen I (2006) Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther 110(3):415–432
Jacobson KA, Costanzi S, Ohno M et al (2004) Molecular recognition at purine and pyrimidine nucleotide (P2) receptors. Curr Top Med Chem 4(8):805–819
King BF, Townsend-Nicholson A, Burnstock G (1998) Metabotropic receptors for ATP and UTP: exploring the correspondence between native and recombinant nucleotide receptors. Trends Pharmacol Sci 19(12):506–514
Harden TK, Lazarowski ER, Boucher RC (1997) Release, metabolism and interconversion of adenine and uridine nucleotides: implications for G protein-coupled P2 receptor agonist selectivity. Trends Pharmacol Sci 18(2):43–46
Akbar GK, Dasari VR, Webb TE et al (1996) Molecular cloning of a novel P2 purinoceptor from human erythroleukemia cells. J Biol Chem 271(31):18363–18367
Li Q, Schachter JB, Harden TK, Nicholas RA (1997) The 6H1 orphan receptor, claimed to be the p2y5 receptor, does not mediate nucleotide-promoted second messenger responses. Biochem Biophys Res Commun 236(2):455–460
Webb TE, Kaplan MG, Barnard EA (1996) Identification of 6H1 as a P2Y purinoceptor: P2Y5. Biochem Biophys Res Commun 219(1):105–110
Yokomizo T, Kato K, Terawaki K, Izumi T, Shimizu T (2000) A second leukotriene B(4) receptor, BLT2. A new therapeutic target in inflammation and immunological disorders. J Exp Med 192(3):421–432
Joost P, Methner A (2002) Phylogenetic analysis of 277 human G-protein-coupled receptors as a tool for the prediction of orphan receptor ligands. Genome Biol 3(11):RESEARCH0063
Nonaka Y, Hiramoto T, Fujita N (2005) Identification of endogenous surrogate ligands for human P2Y12 receptors by in silico and in vitro methods. Biochem Biophys Res Commun 337(1):281–288
Mellor EA, Frank N, Soler D et al (2003) Expression of the type 2 receptor for cysteinyl leukotrienes (CysLT2R) by human mast cells: functional distinction from CysLT1R. Proc Natl Acad Sci USA 100(20):11589–11593
Mellor EA, Maekawa A, Austen KF, Boyce JA (2001) Cysteinyl leukotriene receptor 1 is also a pyrimidinergic receptor and is expressed by human mast cells. Proc Natl Acad Sci USA 98(14):7964–7969
Mamedova L, Capra V, Accomazzo MR et al (2005) CysLT1 leukotriene receptor antagonists inhibit the effects of nucleotides acting at P2Y receptors. Biochem Pharmacol 71(1–2):115–125
Nomura N, Miyajima N, Sazuka T et al (1994) Prediction of the coding sequences of unidentified human genes. I. The coding sequences of 40 new genes (KIAA0001-KIAA0040) deduced by analysis of randomly sampled cDNA clones from human immature myeloid cell line KG-1. DNA Res 1(1):27–35
Charlton ME, Williams AS, Fogliano M, Sweetnam PM, Duman RS (1997) The isolation and characterization of a novel G protein-coupled receptor regulated by immunologic challenge. Brain Res 764(1–2):141–148
Chambers JK, Macdonald LE, Sarau HM et al (2000) A G protein-coupled receptor for UDP-glucose. J Biol Chem 275(15):10767–10771
Foster CJ, Prosser DM, Agans JM et al (2001) Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest 107(12):1591–1598
Hollopeter G, Jantzen HM, Vincent D et al (2001) Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 409(6817):202–207
Takasaki J, Kamohara M, Saito T et al (2001) Molecular cloning of the platelet P2T(AC) ADP receptor: pharmacological comparison with another ADP receptor, the P2Y(1) receptor. Mol Pharmacol 60(3):432–439
Zhang FL, Luo L, Gustafson E et al (2001) ADP is the cognate ligand for the orphan G protein-coupled receptor SP1999. J Biol Chem 276(11):8608–8615
Communi D, Gonzalez NS, Detheux M et al (2001) Identification of a novel human ADP receptor coupled to G(i). J Biol Chem 276(44):41479–41485
Zhang FL, Luo L, Gustafson E et al (2002) P2Y(13): identification and characterization of a novel Galphai-coupled ADP receptor from human and mouse. J Pharmacol Exp Ther 301(2):705–713
Marteau F, Le Poul E, Communi D et al (2003) Pharmacological characterization of the human P2Y13 receptor. Mol Pharmacol 64(1):104–112
Chhatriwala M, Ravi RG, Patel RI, Boyer JL, Jacobson KA, Harden TK (2004) Induction of novel agonist selectivity for the ADP-activated P2Y1 receptor versus the ADP-activated P2Y12 and P2Y13 receptors by conformational constraint of an ADP analog. J Pharmacol Exp Ther 311(3):1038–1043
Marchese A, Sawzdargo M, Nguyen T et al (1999) Discovery of three novel orphan G-protein-coupled receptors. Genomics 56(1):12–21
Schoneberg T, Schulz A, Grosse R et al (1999) A novel subgroup of class I G-protein-coupled receptors. Biochim Biophys Acta 1446(1–2):57–70
Lee DK, Nguyen T, Lynch KR et al (2001) Discovery and mapping of ten novel G protein-coupled receptor genes. Gene 275(1):83–91
Wittenberger T, Schaller HC, Hellebrand S (2001) An expressed sequence tag (EST) data mining strategy succeeding in the discovery of new G-protein coupled receptors. J Mol Biol 307(3):799–813
Jacobs KA, Collins-Racie LA, Colbert M et al (1997) A genetic selection for isolating cDNAs encoding secreted proteins. Gene 198(1–2):289–296
Sugo T, Tachimoto H, Chikatsu T et al (2006) Identification of a lysophosphatidylserine receptor on mast cells. Biochem Biophys Res Commun 341(4):1078–1087
Mombaerts P (2001) The human repertoire of odorant receptor genes and pseudogenes. Annu Rev Genomics Hum Genet 2:493–510
Schulz A, Schoneberg T (2003) The structural evolution of a P2Y-like G-protein-coupled receptor. J Biol Chem 278(37):35531–35541
Engemaier E, Rompler H, Schoneberg T, Schulz A (2006) Genomic and supragenomic structure of the nucleotide-like G-protein-coupled receptor GPR34. Genomics 87(2):254–264
Roy SW, Gilbert W (2006) The evolution of spliceosomal introns: patterns, puzzles and progress. Nat Rev Genet 7(3):211–221
Song KY, Hwang CK, Kim CS et al (2007) Translational repression of mouse mu opioid receptor expression via leaky scanning. Nucleic Acids Res 35(5):1501–1513
Rabadan-Diehl C, Martinez A, Volpi S, Subburaju S, Aguilera G (2007) Inhibition of vasopressin V1b receptor translation by upstream open reading frames in the 5′-untranslated region. J Neuroendocrinol 19(4):309–319
Abbracchio MP, Boeynaems JM, Barnard EA et al (2003) Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci 24(2):52–55
Costanzi S, Mamedova L, Gao ZG, Jacobson KA (2004) Architecture of P2Y nucleotide receptors: structural comparison based on sequence analysis, mutagenesis, and homology modeling. J Med Chem 47(22):5393–5404
Rompler H, Schulz A, Pitra C et al (2005) The rise and fall of the chemoattractant receptor GPR33. J Biol Chem 280(35):31068–31075
Rompler H, Yu HT, Arnold A, Orth A, Schoneberg T (2006) Functional consequences of naturally occurring DRY motif variants in the mammalian chemoattractant receptor GPR33. Genomics 87(6):724–732
Sangkuhl K, Rompler H, Busch W, Karges B, Schoneberg T (2005) Nephrogenic diabetes insipidus caused by mutation of Tyr205: a key residue of V2 vasopressin receptor function. Hum Mutat 25(5):505
Muller T, Bayer H, Myrtek D et al (2005) The P2Y14 receptor of airway epithelial cells: coupling to intracellular Ca2+ and IL-8 secretion. Am J Respir Cell Mol Biol 33(6):601–609
Offermanns S, Simon MI (1995) G alpha 15 and G alpha 16 couple a wide variety of receptors to phospholipase C. J Biol Chem 270(25):15175–15180
Fumagalli M, Trincavelli L, Lecca D, Martini C, Ciana P, Abbracchio MP (2004) Cloning, pharmacological characterisation and distribution of the rat G-protein-coupled P2Y(13) receptor. Biochem Pharmacol 68(1):113–124
Kostenis E, Waelbroeck M, Milligan G (2005) Techniques: promiscuous Galpha proteins in basic research and drug discovery. Trends Pharmacol Sci 26(11):595–602
Conklin BR, Farfel Z, Lustig KD, Julius D, Bourne HR (1993) Substitution of three amino acids switches receptor specificity of Gq alpha to that of Gi alpha. Nature 363(6426):274–276
Ko H, Fricks I, Ivanov AA, Harden TK, Jacobson KA (2007) Structure-activity relationship of uridine 5′-diphosphoglucose analogues as agonists of the human P2Y14 receptor. J Med Chem 50(9):2030–2039
Pausch MH (1997) G-protein-coupled receptors in Saccharomyces cerevisiae: high-throughput screening assays for drug discovery. Trends Biotechnol 15(12):487–494
Price LA, Kajkowski EM, Hadcock JR, Ozenberger BA, Pausch MH (1995) Functional coupling of a mammalian somatostatin receptor to the yeast pheromone response pathway. Mol Cell Biol 15(11):6188–6195
Zhong X, Malhotra R, Guidotti G (2000) Regulation of yeast ectoapyrase ynd1p activity by activator subunit Vma13p of vacuolar H+-ATPase. J Biol Chem 275(45):35592–35599
Pausch MH, Lai M, Tseng E, Paulsen J, Bates B, Kwak S (2004) Functional expression of human and mouse P2Y12 receptors in Saccharomyces cerevisiae. Biochem Biophys Res Commun 324(1):171–177
Ault AD, Broach JR (2006) Creation of GPCR-based chemical sensors by directed evolution in yeast. Protein Eng Des Sel 19(1):1–8
Ding Z, Kim S, Kunapuli SP (2006) Identification of a potent inverse agonist at a constitutively active mutant of human P2Y12 receptor. Mol Pharmacol 69(1):338–345
Andre P, Delaney SM, LaRocca T et al (2003) P2Y12 regulates platelet adhesion/activation, thrombus growth, and thrombus stability in injured arteries. J Clin Invest 112(3):398–406
Haynes SE, Hollopeter G, Yang G et al (2006) The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci 9(12):1512–1519
Schoneberg T, Schulz A, Biebermann H, Hermsdorf T, Rompler H, Sangkuhl K (2004) Mutant G-protein-coupled receptors as a cause of human diseases. Pharmacol Ther 104(3):173–206
Remijn JA, IJsseldijk MJ, Strunk AL et al (2007) Novel molecular defect in the platelet ADP receptor P2Y12 of a patient with haemorrhagic diathesis. Clin Chem Lab Med 45(2):187–189
Shiraga M, Miyata S, Kato H et al (2005) Impaired platelet function in a patient with P2Y12 deficiency caused by a mutation in the translation initiation codon. J Thromb Haemost 3(10):2315–2323
Cattaneo M, Zighetti ML, Lombardi R et al (2003) Molecular bases of defective signal transduction in the platelet P2Y12 receptor of a patient with congenital bleeding. Proc Natl Acad Sci USA 100(4):1978–1983
Fontana P, Dupont A, Gandrille S et al (2003) Adenosine diphosphate-induced platelet aggregation is associated with P2Y12 gene sequence variations in healthy subjects. Circulation 108(8):989–995
Fontana P, Gaussem P, Aiach M, Fiessinger JN, Emmerich J, Reny JL (2003) P2Y12 H2 haplotype is associated with peripheral arterial disease: a case-control study. Circulation 108(24):2971–2973
Schettert IT, Pereira AC, Lopes NH, Hueb WA, Krieger JE (2006) Association between platelet P2Y12 haplotype and risk of cardiovascular events in chronic coronary disease. Thromb Res 118(6):679–683
Ziegler S, Schillinger M, Funk M et al (2005) Association of a functional polymorphism in the clopidogrel target receptor gene, P2Y12, and the risk for ischemic cerebrovascular events in patients with peripheral artery disease. Stroke 36(7):1394–1399
International HapMap Consortium (2005) A haplotype map of the human genome. Nature 437(7063):1299–1320
Bamshad M, Wooding SP (2003) Signatures of natural selection in the human genome. Nat Rev Genet 4(2):99–111
Nielsen R, Bustamante C, Clark AG et al (2005) A scan for positively selected genes in the genomes of humans and chimpanzees. PLoS Biol 3(6):e170
Nielsen R, Williamson S, Kim Y, Hubisz MJ, Clark AG, Bustamante C (2005) Genomic scans for selective sweeps using SNP data. Genome Res 15(11):1566–1575
Tang K, Thornton KR, Stoneking M (2007) A new approach for using genome scans to detect recent positive selection in the human genome. PLoS Biol 5(7):e171
Kimura R, Fujimoto A, Tokunaga K, Ohashi J (2007) A practical genome scan for population-specific strong selective sweeps that have reached fixation. PLoS ONE 2:e286
Joensuu T, Hamalainen R, Lehesjoki AE, de la Chapelle A, Sankila EM (2000) A sequence-ready map of the Usher syndrome type III critical region on chromosome 3q. Genomics 63(3):409–416
Bedard A, Tremblay P, Chernomoretz A, Vallieres L (2007) Identification of genes preferentially expressed by microglia and upregulated during cuprizone-induced inflammation. Glia 55(8):777–789
Ballesteros JA, Weinstein H (1995) Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci 25:366–428
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Schöneberg, T., Hermsdorf, T., Engemaier, E. et al. Structural and functional evolution of the P2Y12-like receptor group. Purinergic Signalling 3, 255–268 (2007). https://doi.org/10.1007/s11302-007-9064-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-007-9064-0