Abstract
The adenosine A2B receptor is the least well characterized of the four adenosine subtypes due to the lack of potent and selective agonists and antagonists. Despite the widespread distribution of A2B receptor mRNA, little information is available with regard to their function. The characterization of A2B receptors, through radioligand binding studies, has been performed, until now, by using low-affinity and non-selective antagonists like 1,3-dipropyl-8-cyclopentylxanthine ([3H]DPCPX),(4-(2-[7-amino-2-(2-furyl)-[1,2,4]triazolo-[2,3-a][1,3,5]triazin-5-ylamino]ethyl)-phenol ([3H]ZM 241385) and 3-(3,4-aminobenzyl)-8-(4-oxyacetate)phenyl-1-propyl-xanthine ([125I]ABOPX). Recently, high-affinity radioligands for A2B receptors, [N-(4-cyanophenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)-phenoxy]acetamide ([3H]MRS 1754), N-(2-(2-Phenyl-6-[4-(2,2,3,3-tetratritrio-3-phenylpropyl)-piperazine-1-carbonyl]-7H-pyrrolo[2,3-d]pyrimidin-4-ylamino)-ethyl)-acetamide ([3H]OSIP339391) and N-benzo[1,3]dioxol-5-yl-2-[5-(1,3-dipropyl-2,6-dioxo-2,3,6,7-tetrahydro-1H-purin-8-yl)-1-methyl-1H-pyrazol-3-yloxy]-acetamide] ([3H]MRE 2029F20), have been introduced. This minireview offers an overview of these recently developed radioligands and the most important applications of drugs towards A2B receptors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adenosine, an ubiquitous nucleoside released from metabolically active or stressed cells, is known to act as an important regulatory molecule through activation of cell surface receptors named A1, A2A, A2B and A3, all of which belong to the G protein-coupled superfamily of receptors [1]. In particular A1 and A3 inhibit, through Gi proteins, adenylyl cyclase activity, whereas A2A and A2B stimulate, via Gs proteins, this enzyme. Collectively, these receptors are widespread on virtually every organ and tissue and represent promising drug targets for the pharmacological intervention in many pathophysiological conditions that are believed to be associated with changes of adenosine levels such as asthma, neurodegenerative disorders, chronic inflammatory diseases and cancer [2, 3]. It has been suggested that adenosine receptors act as “sensors” and that extracellular adenosine acts as a “reporter” of metabolic changes in the local tissue environment [4, 5]. Therefore, under normal conditions adenosine, which is continuously produced intracellularly and extracellularly and maintained at low intracellular levels (about 100 nM) by adenosine kinase and adenosine deaminase, interacts with the high-affinity A1 and A2A receptor subtypes. In hypoxic, ischaemic or inflamed conditions the intracellular production of adenosine is increased to very high micromolar concentrations and transported across cell membranes by specific agents finally leading to the stepwise activation of all adenosine receptors, including the low-affinity A2B and A3 subtypes [6].
Whilst for A1, A2A and A3 adenosine subtypes good agonists and antagonists have been synthesized allowing a plethora of binding and functional studies, no high-affinity analogues have been identified for the A2B receptors until a couple of years ago, and neither high-affinity nor selective antagonists had been introduced until 2001 [3, 7, 8]. Therefore studies concerning A2B receptors rely on mRNA distribution, cloning and functional assays performed with not very selective molecules [9]. However considering the potential therapeutic applications of A2B ligands, many efforts have been made in the last few years to search for potent and selective antagonists versus this receptor subtype [10] and the recent discovery of novel radiolabelled A2B antagonists will be useful to the scientific community for the exploration and characterization of the pathophysiological role of A2B receptors.
A2B adenosine receptors
A2B receptors were distinguished from A2A in 1983 [11] due to the high and low affinity of adenosine in the stimulation of cAMP levels in rat striatum and were cloned for the first time in 1992 from human hippocampus [12] and rat brain [13, 14] and subsequently from mouse bone marrow-derived mast cells [15]. The distribution of A2B receptors has been detected on practically every cell in different species and relies on the reverse transcription polymerase chain reaction technique. A2B messenger RNA has been found at low levels in all rat brain regions evaluated, aorta, stomach, testis, skeletal muscle, jejunum, kidney, heart, skin, spleen and liver whilst high levels have been detected in the proximal colon [6]. A2B are coupled to different signalling pathways such as stimulation of adenylate cyclase through Gs proteins and accumulation of intracellular calcium. This pathway is related to different mechanisms such as potentiation of a P-type calcium current in hippocampal neurons [16], activation of a calcium channel, Gs protein-coupled, in human erythroleukemia cells [17] or involvement of phospholipase C in HMC1 cells [18]. More recently, it has also been suggested that they are involved in activation of extracellular regulated kinases 1/2 (ERK1/2) at physiologically normal concentrations [19, 20]. Functional studies have suggested the presence of A2B receptors in airway smooth muscle, fibroblasts, vasculature, mast cells, macrophages, endothelium, haematopoietic cells and intestinal epithelial cells. In particular, they have been reported to be involved in stimulation of cell proliferation, differentiation and migration of retinal endothelial cells, where A2B antagonists may offer a way to inhibit retinal angiogenesis and provide a novel therapeutic approach for treatment of diseases associated with aberrant neovascularization, such as diabetic retinopathy [21, 22]. In macrophages it has been reported that their up-regulation induced by interferon gamma (IFN-γ) would lead to the cAMP-mediated down-regulation of MHC class II molecules and other macrophage activities. This may constitute an important mechanism of cell deactivation at the inflammatory foci [23]. In human mast cells 5′-N-ethyl-carboxamidoadenosine (NECA) induced interleukin-8 secretion through the involvement of p38 mitogen-activated protein kinase and extracellular signal-regulated protein kinase kinase and this effect was blocked by 3-isobutyl-8-pyrrolidinoxanthine (IPDX) suggesting a basis for the development of new antiasthmatic drugs [24–26]. The recent discovery that A2B receptors are also functionally active on human airway smooth muscle cells to enhance cytokine and chemokine release [27] and on lung fibroblasts where they promote differentiation to a myofibroblast phenotype [28] supports the view that this subtype may be involved in airway wall remodelling and in asthma [29]. Interestingly a role of A2B receptors has also been observed in the analgesic effects of caffeine in an acute animal model of nociception, suggesting that specific A2B antagonists might be valuable adjuvant drugs for opioid analgesia with minimal side effects [30]. The high expression of A2B receptors in different parts of the intestinal tract raised great interest in defining their function. It has been suggested that they play a role in epithelial secretion with potential relevance to diarrhoeal processes [31]. Moreover A2B receptors are involved in coronary flow regulation [32], stimulation of proangiogenic factors [33–35] and they have been proposed as targets to control cell growth and proliferation in a human breast cancer cell line [36]. All together these effects suggest a possible role of the A2B subtype in the modulation of inflammatory processes involved in asthma, tumour growth, tissue injury, ischaemia and pain [37, 38]. Targeting of the A2B receptor protein to specific cells or tissues is crucial in order to understand their role in pathophysiological conditions. Unfortunately, the lack of selective agonists and antagonists has hampered the pharmacological characterization of this receptor system. Only recently Ijzerman’s group developed new, high-potency A2B agonists with an improved selectivity profile compared to the reference agonist NECA, and the discovery of the first A2B partial agonist that appears very promising for future functional studies [39]. The characterization of A2B receptors, therefore, often was based on the lack of effectiveness of compounds that are potent and selective agonists of other receptor subtypes. The agonist CGS 21680, for example, has been useful in differentiating between A2A and A2B receptors [40]. However, pharmacological characterization of receptors based on apparent agonist potencies is far from ideal, because it depends not only on agonist binding to the receptor but also on multiple processes involved in signal transduction. Recently, based on functional assays using novel A2B selective antagonists, significant progress has been made in the understanding of the molecular pharmacology and physiology of A2B adenosine receptors and novel progress in this field is supposed to be increased by the introduction of the tritiated radiolabelled form of some new A2B antagonists named [3H]MRS 1754, [3H] OSIP339391 and [3H]MRE 2029-F20 [41–43].
Novel antagonist radioligands to A2B adenosine subtype
The characterization of A2B receptors, through radioligand binding studies, has been performed, until now, by using low-affinity and non-selective antagonists like [3H]DPCPX, [3H]ZM 241385 and [125I]ABOPX, which, as a consequence of their low affinity, display a rapid dissociation rate from the receptor [44]. In addition, since these ligands are non-selective their utility in native systems is hampered as many tissues and cell lines express several adenosine subtypes.
Xanthines, including the natural derivatives theophylline and caffeine, are the natural non-selective antagonists of adenosine and they are able to bind to all four adenosine receptor subtypes A1, A2A, A2B and A3 [6]. Therefore the xanthine core has been maintained and modified in order to develop new A2B drugs. This approach led to the discovery of new molecules introduced as high-affinity radioligands for A2B receptors named [3H]MRS 1754, [3H]OSIP339391 and [3H]MRE 2029-F20 [41–43].
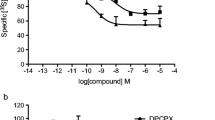
MRS1754, which belongs to a series of amides derived from the 8-phenyl xanthine molecules [45], was the first antagonist proposed in radiolabelled form for the labelling of A2B receptors [41]. MRS1754 was shown to be a potent and selective antagonist. Radioligand binding studies showed that it binds to recombinant A2B receptors in membranes of stably transfected HEK 293 cells. Specific binding was saturable, competitive, and followed a one-site model, with a KD value of 1.13 nM and a Bmax value of 10.9 pmol/mg protein. Specific binding of [3H]MRS 1754, at the KD value, was >70% of total binding. The affinity calculated from association and dissociation binding constants was 1.22 nM. Binding to membranes expressing rat and human A1 and A3 adenosine receptors was not significant, and binding in membranes of HEK 293 cells expressing human A2A receptors was of low affinity (KD > 50 nM). The pharmacological profile in competition experiments with [3H]MRS 1754 was consistent with the structure-activity relationship for agonists and antagonists at A2B receptors. In spite of the high affinity and selectivity, this radioligand has not been used in cells and tissues endogenously expressing A2B receptors.
Some years later a pyrrolopyrimidine compound [3H] OSIP339391 was introduced as a high-affinity and selective radioligand to A2B receptors [42]. OSIP339391 had a selectivity of greater than 70-fold for A2B receptors over other human adenosine receptor subtypes. Using membranes from HEK 293 cells expressing the human recombinant A2B receptor, [3H]OSIP339391 was characterized in kinetic, saturation and competition binding experiments. From the association and dissociation rate studies, the affinity was 0.41 nM and in close agreement with that found in saturation binding experiments (0.17 nM). In competition binding studies of [3H]OSIP339391, the affinity of a range of agonists and antagonists was consistent with previously reported data. However, also in this case, its suitability to label endogenous A2B receptors cannot be assessed due to the lack of binding studies in cells or tissues.
Our research group has identified a series of 8-pyrazole xanthine derivatives as potent and selective human A2B adenosine antagonists [46], and a radiolabelled form of one compound of this series, [3H]MRE 2029-F20 [47], was used as new pharmacological tool to describe the comparison between human recombinant A2B receptors stably transfected in HEK 293 cells and endogenous receptors present in human neutrophils and lymphocytes [43]. [3H]MRE 2029-F20 showed high affinity and selectivity for hA2B versus hA1, hA2A and hA3 subtypes. The selectivity of MRE 2029-F20 for the human A2B over A1, A2A and A3 receptors was evaluated in radioligand binding assays by using [3H] DPCPX, [3H]ZM 241385 and [3H]MRE 3008-F20, respectively. MRE 2029-F20 displays low affinity for the human A1 receptor (Ki = 245 ± 31 nM) and no significant affinity for the human A2A and A3 subtypes (Ki >1,000 nM). This indicates that MRE 2029-F20 is 88- and more than 300-fold selective for the A2B over A1, A2A and A3 subtypes, respectively, which means a range of selectivity similar to [3H]MRS 1754 (selectivity of 210-, 260- and 290-fold for A2B over A1, A2A and A3 subtypes, respectively) and slightly better than [3H]OSIP339391 (selectivity of 70-fold for A2B over A1, A2A and A3 subtypes, respectively).
[3H]MRE 2029-F20 bound specifically to the hA2B receptor stably transfected in human embryonic kidney (HEK) 293 cells with KD of 2.8 nM and Bmax of 450 fmol/mg of protein. Saturation experiments of [3H]MRE 2029-F20 binding in human neutrophils and lymphocytes detected a single high-affinity binding site with KD values of 2.4 and 2.7 nM, respectively, and Bmax values of 79 and 54 fmol/mg of protein, respectively, in agreement with real-time reverse transcription polymerase chain reaction studies showing the presence of A2B mRNA. The rank order of potency of typical adenosine ligands with recombinant hA2B receptors was consistent with that typically found for interactions with the A2B subtype and was also similar in peripheral blood cells. NECA stimulated cAMP accumulation in both hA2BHEK 293 and native cells, whereas phospholipase C activation was observed in recombinant receptors and endogenous subtypes expressed in neutrophils but not in lymphocytes. MRE 2029-F20 was revealed to be a potent antagonist in counteracting the agonist effect in both signal transduction pathways. In conclusion, [3H] MRE 2029-F20 was revealed to be a selective and highaffinity radioligand for the hA2B adenosine subtype and may be used to quantify A2B endogenous receptors such as those present in neutrophils and lymphocytes that represent inflammatory cells potentially involved in the exacerbation of asthma and other inflammatory processes in which A2B receptors are thought to be involved [29]. This was the first paper reporting both binding and functional studies in recombinant and native receptors. [3H]MRE 2029-F20 has been used also to evaluate the effects of novel and recognised compounds at human recombinant A2B adenosine receptors expressed in Chinese hamster ovary, in HEK 293 and at endogenous A2B receptors in human mast cells (HMC-1). Saturation binding experiments performed using [3H]MRE 2029-F20 revealed a single class of binding sites in hA2BCHO, hA2BHEK 293 and HMC-1 cells with KD of 1.65, 2.83, 2.62 nM and Bmax of 36, 475 and 128 fmol/mg protein, respectively. Moreover, the compounds tested were able to decrease NECA-stimulated and also forskolin-stimulated cAMP production, revealing them to be novel antagonists with an inverse agonist activity in recombinant and native human A2B receptors [48].
Conclusions
In recent years remarkable attention has been paid to A2B receptors due to their supposed role in important pathological states such as asthma, diarrhoeal processes, diabetic retinopathy, vasodilation, neoangiogenesis and nociception. For many years their study has been strongly limited by the lack of good and selective pharmacological probes and many efforts have been focused by chemists and biologists on the synthesis and testing of new selective ligands. The data presented in this review clearly underline the significant progress made in the field of A2B receptor antagonists. Novel high-affinity and selective antagonist radioligands have been recently synthesized that will allow in the future the characterization of this adenosine subtype in endogenous systems (Table 1).
Abbreviations
- ABOPX:
-
3-(3,4-aminobenzyl)-8-(4-oxyacetate)phenyl-1-propyl-xanthine
- DPCPX:
-
1,3-dipropyl-8-cyclopentyl-xanthine
- IB-MECA:
-
N6-(3-iodo-benzyl) adenosine-5′N-methyluronamide
- IPDX:
-
3-isobutyl-8-pyrrolidinoxanthine
- MRE 3008F20:
-
5-N-(4-methoxyphenyl-carbamoyl)amino-8-prpyl-2(2furyl)-pyrazolo-[4,3e]-1,2,4-triazolo [1,5-c]pyrimidine
- MRE 2029-F20:
-
N-benzo[1,3]dioxol-5-yl-2-[5-(1,3-dipropyl-2,6-dioxo-2,3,6,7-tetrahydro-1H-purin-8-yl)-1-methyl-1H-pyrazol-3-yloxy]-acetamide]
- MRS 1754:
-
[N-(4-cyanophenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)-phenoxy]acetamide
- NECA:
-
5′N-ethylcarboxamidoadenosine
- OSIP339391:
-
N-(2-(2-Phenyl-6-[4-(2,2,3,3-tetratritrio-3-phenylpropyl)-piperazine-1-carbonyl]-7H-pyrrolo[2,3-d]pyrimidin-4-ylamino)-ethyl)-acetamide
- SCH 58261:
-
7-(2-phenylethyl)-2-(2-furyl)-pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]-pyrimidine
- hA2BHEK 293 cells:
-
Human embryonic kidney-293 cells transfected with human adenosine A2B receptor
- PLC:
-
Phospholipase C
- ZM 241385:
-
(4-(2-[7-amino-2-(2-furyl)-[1,2,4]triazolo-[2,3-a][1,3,5]triazin-5-ylamino] ethyl)-phenol
References
Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J (2001) International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53:527′52
Fredholm BB (2003) Adenosine receptors as targets for drug development. Drug News Perspect 16:283′89
Jacobson KA, Gao Z (2006) Adenosine receptors as therapeutic targets. Nat Rev 5:247′64
Sitkovsky M, Ohta A (2005) The ‘danger′sensors that STOP the immune response: the A2 adenosine receptors? Trends Immunol 26:299′04
Newby AC (1984) Adenosine and the concept of retaliatory metabolites. Trends Biochem Sci 9:42′4
Ralevic V, Burnstock G (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50:413′92
Cristalli G, Volpini R (eds) (2003) Adenosine receptors: chemistry and pharmacology. Curr Top Med Chem 3(4):1′69
Volpini R, Costanzi S, Vittori S, Cristalli G, Klotz KN (2003) Medicinal chemistry and pharmacology of A2B adenosine receptors. Curr Top Med Chem 3:427′43
Feoktistov I, Biaggioni I (1997) Adenosine A2B receptors. Pharmacol Rev 49:381′02
Cacciari B, Pastorin G, Bolcato C et al (2005) A2B adenosine receptor antagonists: recent developments. Mini Rev Med Chem 5:1053′060
Daly JW, Butts-Lamb P, Padgett W (1983) Subclasses of adenosine receptors in the central nervous system: interactions with caffeine and related methylxanthines. Cell Mol Neurobiol 3:69
Pierce KD, Furlong TJ, Selbie LA, Shine J (1992) Molecular cloning of an adenosine receptor from human brain. Biochem Biophys Res Commun 187:86′3
Rivkees SA, Reppert SM (1992) RFL9 encodes an A2b-adenosine receptor. Mol Endocrinol 6:1598′604
Stehle JH, Rivkees SA, Lee JJ, Weaver DR, Deeds JD, Reppert SM (1992) Molecular cloning and expression of the cDNA for a novel A2-adenosine receptor subtype. Mol Endocrinol 6:384′93
Marquardt DL, Walker LL, Heinemann S (1994) Cloning of two adenosine receptor subtypes from mouse bone marrow-derived mast cells. J Immunol 152:4508′515
Mogul DJ, Adams ME, Fox AP (1993) Differential activation of adenosine receptors decreases N-type but potentiates P-type Ca2+ current in hippocampal CA3 neurons. Neuron 10:327′34
Feoktistov I, Murray JJ, Biaggioni I (1994) Positive modulation of intracellular Ca2+ levels by adenosine A2b receptors, prostacyclin and prostaglandin E1 via a cholera toxin-sensitive mechanism in human erythroleukemia cells. Mol Pharmacol 45:1160′167
Feoktistov I, Biaggioni I (1995) Adenosine A2b receptors evoke interleukin-8 secretion in human mast cells. An enprofylline-sensitive mechanism with implications for asthma. J Clin Invest 96:1979′986
Gao Z, Chen T, Weber MJ, Linden J (1999) A2B adenosine and P2Y2 receptors stimulate mitogen-activated protein kinase in human embryonic kidney-293 cells. J Biol Chem 274:5972′980
Schulte G, Fredholm BB (2000) Human adenosine A(1), A(2A), A(2B), and A(3) receptors expressed in Chinese hamster ovary cells all mediate the phosphorylation of extracellular-regulated kinase 1/2. Mol Pharmacol 58:477′82
Grant MB, Tarnuzzer RW, Caballero S et al (1999) Adenosine receptor activation induces vascular endothelial growth factor in human retinal endothelial cells. Circ Res 85:699′06
Grant MB, Davis MI, Caballero S, Feoktistov I, Biaggioni I, Belardinelli L (2001) Proliferation, migration, and ERK activation in human retinal endothelial cells through A(2B) adenosine receptor stimulation. Invest Ophthalmol Vis Sci 42:2068′073
Xaus J, Mirabet M, Lloberas et al (1999) IFN-γ up-regulates the A2B adenosine receptor expression in macrophages: a mechanism of macrophage deactivation. J Immunol 162:3607′614
Feoktistov I, Goldstein AE, Biaggioni I (1999) Role of p38 mitogen-activated protein kinase and extracellular signal-regulated protein kinase kinase in adenosine A2B receptor-mediated interleukin-8 production in human mast cells. Mol Pharmacol 55:726′34
Feoktistov I, Garland EM, Goldstein AE, Zeng D, Belardinelli L, Wells JN, Biaggioni I (2001) Inhibition of human mast cell activation with the novel selective adenosine A(2B) receptor antagonist 3-isobutyl-8-pyrrolidinoxanthine (IPDX). Biochem Pharmacol 62:1163′173
Feoktistov I, Ryzhov S, Goldstein AE, Biaggioni I (2003) Mast cell-mediated stimulation of angiogenesis: cooperative interaction between A2B and A3 adenosine receptors. Circ Res 92:485′92
Zhong H, Belardinelli L, Maa T, Feoktistov I, Biaggioni I, Zeng D (2004) A(2B) adenosine receptors increase cytokine release by bronchial smooth muscle cells. Am J Respir Cell Mol Biol 30:118′25
Zhong H, Belardinelli L, Maa T, Zeng D (2005) Synergy between A2B adenosine receptors and hypoxia in activating human lung fibroblasts. Am J Respir Cell Mol Biol 32:2′
Holgate S (2005) The identification of the adenosine A2B receptor as a novel therapeutic target in asthma. Br J Pharmacol 145:1009′015
Abo-Salem OM, Hayallah AM, Bilkei-Gorzo A, Filipek B, Zimmer A, Muller CE (2004) Antinociceptive effects of novel A2B adenosine receptor antagonists. J Pharmacol Exp Ther 308:358′66
Strohmeier GR, Reppert SM, Lencer WI, Madara JL (1995) The A2b adenosine receptor mediates cAMP responses to adenosine receptor agonists in human intestinal epithelia. J Biol Chem 270:2387′394
Talukder MA, Morrison RR, Ledent C, Mustafa SJ (2003) Endogenous adenosine increases coronary flow by activation of both A2A and A2B receptors in mice. J Cardiovasc Pharmacol 41:562′70
Feoktistov I, Goldstein AE, Ryzhov S, Zeng D, Belardinelli L, Voyno-Yasenetskaya T, Biaggioni I (2002) Differential expression of adenosine receptors in human endothelial cells: role of A2B receptors in angiogenic factor regulation. Circ Res 90:531′38
Afzal A, Shaw LC, Caballero S, Spoerri PE, Lewin AS, Zeng D, Belardinelli L, Grant MB (2003) Reduction in preretinal neovascularization by ribozymes that cleave the A2B adenosine receptor mRNA. Circ Res 93:500′06
Fiebich BL, Biber K, Gyufko K, Berger M, Bauer J, van Calker D (1996) Adenosine A2b receptors mediate an increase in interleukin (IL)-6 mRNA and IL-6 protein synthesis in human astroglioma cells. J Neurochem 66(4):1426′431
Panjehpour M, Castro M, Klotz KN (2005) Human breast cancer cell line MDA-MB-231 expresses endogenous A2B adenosine receptors mediating a Ca2+ signal. Br J Pharmacol 145:211′18
Livingston M, Heaney LG, Ennis M (2004) Adenosine, inflammation and asthma—a review. Inflamm Res 53:171′78
Spicuzza L, Bonfiglio C, Polosa R (2003) Research applications and implications of adenosine in diseased airways. Trends Pharmacol Sci 24:409′13
Beukers MW, Chang LC, Kunzel J, Mulder-Krieger T et al (2004) New, non-adenosine, high potency agonists for the human adenosine A2B receptor with an improved selectivity profile compared to the reference agonist N-ethylcarboxamidoadenosine. J Med Chem 47:3707′709
Feoktistov I, Biaggioni I (1998) Pharmacological characterization of adenosine A2B receptors: studies in human mast cells co-expressing A2A and A2B adenosine receptor subtypes. Biochem Pharmacol 55:627′33
Ji X, Kim YC, Ahern DG, Linden J, Jacobson KA (2001) [3H]MRS 1754, a selective antagonist radioligand for A(2B) adenosine receptors. Biochem Pharmacol 61:657′63
Stewart M, Steinig AG, Ma C, Song JP, McKibben B, Castelhano AL, MacLennan SJ (2004) [3H]OSIP339391, a selective, novel, and high affinity antagonist radioligand for adenosine A2B receptors. Biochem Pharmacol 68:305′12
Gessi S, Varani K, Merighi S, Cattabriga E, Pancaldi C, Szabadkai Y, Rizzato R, Klotz KN, Leung E, Mac Lennan S, Baraldi PG, Borea PA (2005) Expression, pharmacological profile, and functional coupling of A2B receptors in a recombinant system and in peripheral blood cells by using a novel selective antagonist radioligand [3H]MRE 2029-F20. Mol Pharmacol 67:2137′147
Linden J, Thai T, Figler H, Jin X, Robeva A (1999) Characterization of human A(2B) adenosine receptors: radioligand binding, western blotting, and coupling to G(q) in human embryonic kidney 293 cells and HMC-1 mast cells. Mol Pharmacol 56:705′13
Kim Y, Ji X, Melman N, Linden J et al (2000) Aniline derivatives of an 8-phenylxanthine carboxylic congener are highly potent and selective antagonists at human A(2B) adenosine receptors. J Med Chem 43:1165′172
Baraldi PG, Tabrizi MA, Preti D et al (2004) Design, synthesis, and biological evaluation of new 8-heterocyclic xanthine derivatives as highly potent and selective human A2B adenosine receptor antagonists. J Med Chem 47:1434′447
Baraldi PG, Tabrizi MA, Preti D et al (2004) [3H]-MRE 2029-F20, a selective antagonist radioligand for the human A2B adenosine receptors. Bioorg Med Chem Lett 14:3607′610
Varani K, Gessi S, Merighi S et al (2005) Pharmacological characterization of novel adenosine ligands in recombinant and native human A2B receptors. Biochem Pharmacol 70(11):1601′612
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Gessi, S., Varani, K., Merighi, S. et al. Novel selective antagonist radioligands for the pharmacological study of A2B adenosine receptors. Purinergic Signalling 2, 583–588 (2006). https://doi.org/10.1007/s11302-006-9019-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-006-9019-x