Abstract
The feeding ecology of Great Cormorants (Phalacrocorax carbo) during the breeding season in the Kano River basin, central Japan, was examined to clarify the trophic relationship between the cormorants and ayu (Plecoglossus altivelis altivelis) reared for mass release in the river. The ayu was most frequently found in stomachs of cormorants culled during the breeding season, despite relatively poor catch in the year-round fish fauna research in the watershed. Carbon and nitrogen stable isotope ratios of some ayu individuals extracted from the stomachs of the culled cormorants were similar to the isotopic values of ayu caught in the watershed, whereas the other stomach-content ayu showed peculiarly high nitrogen isotopic values, clearly distinct from the values of the ayu caught in the watershed, and overlapped with the values of mass-release ayu. Furthermore, isotopic values of past diets inferred by the isotope analysis of livers of the culled cormorants were closer to the values of the mass-release ayu, relative to the past diet values inferred by the analysis of the cormorant muscles. This suggests that the food supply from the mass-release ayu had increased in the breeding season, since the isotopic turnover rate is faster in livers than in muscles. The huge number of formula-fed ayu released in the watershed create an anthropogenic food chain which is assumed to significantly support the breeding of the cormorants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Great Cormorant (Phalacrocorax carbo) is a piscivorous bird inhabiting river basins and coastal seas in Eurasia, Africa, Australia and the North America (Simpson and Day 2004). The number of cormorants in Japan had decreased in the mid 20th century, with a minimum population of ≤ 3000 individuals within the country in 1971 (Fukuda et al. 2002). However, the population has recovered since the mid 1970s, and has caused serious problems such as decimation of freshwater fish stocks through feeding and forests through showering of excreta (Fukuda et al. 2002, Kameda et al. 2002). The National Federation of Inlandwater Fisheries Cooperatives (2004) estimated the total financial damage to domestic freshwater fisheries at 4.56 billion yen. As a result, culling of cormorants is now practiced in various areas in Japan.
In Japan, the most serious problem regarding the cormorants is the feeding damage to the stock of ayu (Plecoglossus altivelis altivelis), one of the most important species for the inland fisheries (Takahashi et al. 2006). The ayu is a diadromous species with a short life span of usually 1 year (Nishida 1989). Adults spawn in rivers mainly from October to December. Hatched larvae flow down to the sea and juveniles swim back up the rivers in the following spring (Nishida 1989). Throughout Japan, a huge amount of juvenile ayu reared in seed production facilities are released in spring and summer, totalling 136,964,000 individuals in 2008 (Ministry of Agriculture, Forestry and Fisheries 2010). Some studies found that stomach contents of culled cormorants consisted largely of ayu in the released season, and the cormorants gathered around the place where the ayu were released (Fisheries Agency 2003; Iguchi et al. 2008). Therefore, the cormorants are generally supposed to prey on these mass-release ayu individuals in the released season.
However, actually little is known about the trophic relationship between the cormorants and the mass-release ayu, since it is difficult to identify the origin of the ayu preyed on by the cormorants, which would determine the ratio of the wild ayu to the mass-release ayu. To evaluate significance and effectiveness of the culling of the cormorants, it is essential to precisely understand their trophic relationship with the ayu. Thus in this study, we examined the feeding ecology of the cormorant in the Kano River basin, with emphasis on food supply from the mass-release ayu. In particular, we conducted analysis of carbon and nitrogen stable isotope ratios (δ13C and δ15N) to trace the feeding record of the cormorants.
The stable isotope analysis presents time-integrated information on the feeding relationships (Wada et al. 1987; Fry 1988). The δ13C of heterotrophs shows a slight enrichment of < 1.5‰ per trophic level in many cases (Deniro and Epstein 1978; Rau et al. 1983; Post 2002). This conservative nature of δ13C along a food chain can provide information on the carbon sources of higher consumers. On the other hand, the δ15N shows a stepwise enrichment of 3–4‰ per trophic level on average (Deniro and Epstein 1981; Minagawa and Wada 1984). Interpretation of consumers δ15N relative to the δ15N of the primary producer provides a measure of their trophic position that is indicative of “average” trophic function (Levine 1980). The combined use of δ13C and δ15N has clarified the feeding records of the cormorants in various regions of the world (Bearhop et al. 1999; Doucette et al. 2011; Natsumeda et al. 2015).
In the present study, we conducted (1) stomach content analysis of the cormorants culled during the breeding season, (2) year-round research of fish fauna in the Kano River watershed, and (3) stable isotope analysis of the culled cormorants and their prey, to clarify the feeding ecology of the cormorants in the breeding season. In our previous study, we grasped the general overview of habitat use of the cormorants in the Kano River basin by population censuses (Kawabe et al. 2013). Here we considered the trophic relationship between the cormorants and the ayu by comparing the results of these multiple investigations regarding the food habits and the habitat use of the cormorants, and the distribution of their prey fish.
Materials and methods
Sampling and dissection of Great Cormorants culled in a main colony
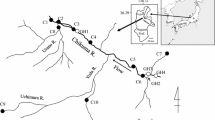
A main colony of Great Cormorants existed in the northernmost area of the Izu Peninsula during the research period (Fig. 1). Shooting of the cormorants in the colony had been performed for culling of the species in the Kano River basin by the Kano River Fisheries Cooperative Association and the Tagata Hunting Club. We collected 73 cormorants there on May 3 and June 20 in 2009, on May 22, May 29 and June 6 in 2010, and on May 28 and June 20 in 2011.
Study area. a The whole region of the Kano River watershed and the Izu Peninsula. Panels b–d show magnifications of areas b–d in the map (a). Great Cormorants Phalacrocorax carbo were shot in a colony (a solid diamond). Throw nets were used for main sampling of fish at 28 stations (shaded circles) and were not used for supplementary sampling at 45 stations (open circles)
We measured wing span of the culled cormorants, and extracted contents of stomachs and gullets by dissection. Hereafter, both the contents of the stomachs and the gullets are called “stomach contents”. The prey species included in the stomach contents were identified to the lowest taxon. We counted the number of individuals and measured the wet weight for each prey species. We also measured body length (standard length) of the stomach-content fish if possible. The standard length of fish is measured from the tip of the snout to the end of the hypural. In this study, academic names and identification of fish species mainly follow Nakabo (2000).
Sampling of fish in the Kano River watershed
The fish in the Kano River watershed were collected by means of throw nets, hand nets and bare hands at 28 stations from July 2008 to December 2009 (Fig. 1). In the watershed, a large quantity of ayu was released mainly during late March to early July every year of our research (Table 1). Therefore, we did not perform the throw-net sampling in the mainstream during this period to avoid causing serious damage to the population of the released ayu. The fish in the mainstream (C1–C7), the western tributaries (W1–W4), and the eastern tributaries (E1–E4) were sampled in July, September, and December in 2008, and spring (March or April) in 2009, while the fish in the northern tributaries (N1–N7) and the northeastern tributaries in the vicinity of Hakone (H1–H6) were sampled in April, July, September, and December in 2009. The use of the throw nets was permitted by Shizuoka Prefecture and all the research was supported by the Kano River Fisheries Cooperative Association.
As supplementary sampling, we also collected fish by means of hand nets, angling, and bare hands at other 45 stations from June to December in 2010. The sampling was performed at five stations in the mainstream (c1–c5), seven stations in the western tributaries (w1–w7), 12 stations in the eastern tributaries (e1–e12), eight stations in the northern tributaries (n1–n8), and 13 stations in the northeastern tributaries (h1–h13).
We collected mass-release ayu released on 15 March 2013 and 16 April 2013 in the watershed. These fish were provided by the fisheries cooperative association. Furthermore, we also collected marine fish, Japanese horse mackerel (Trachurus japonicus), in the river mouth of the Kano River, and Japanese dace (Tribolodon hakonensis) in the river mouth of the Inohzawa River, the southeastern area of the Izu Peninsula. These fish were analyzed for the stable isotope in order to ecologically interpret the isotopic values of the dace extracted from the cormorant stomachs. The mackerel in the Kano River were collected by means of angling. The dace in the Inohzawa River were collected by means of throw nets and frozen at – 20 °C in our previous fish fauna research (Takai et al. 2011).
Stable isotope analysis
Stable isotope analysis was performed on tissues and stomach contents of the culled cormorants, fish collected in rivers, fish reared in the seed production facility, and formula feed used in the seed production. The analysis of the culled cormorants was performed on muscles and livers, and the analysis of the stomach contents was performed on muscles of undigested fish. The riverine fishes and the mass-release ayu were analyzed in the muscle tissue. The formula feed was analyzed in the mixture of dozens of pellets. δ13C of the formula feed was analyzed for the sample saturated with 1 M HCl solution for a day and then dried, in order to eliminate carbonates. The samples were dried at 60 °C and were ground to a fine powder. Subsequently, the tissues were defatted with a chloroform–methanol (2:1) solution in order to eliminate the influence by the variation of the content of lipids that are more depleted in 13C than amino acids and carbohydrates.
In some cases, the stable isotope ratios of the cormorants have been reported for tissues that were not defatted (Mizutani et al. 1991). Therefore, a part of the culled cormorants (n = 30) were analyzed in both the defatted tissue and the untreated tissue of the identical cormorant to examine the change of the isotopic values after the removal of the lipids.
The isotope ratios were measured with a Delta Plus Advantage mass spectrometer coupled with a Flash EA 1112 elemental analyzer (Thermo Fisher Scientific). The isotopic values are expressed as per mil deviations from the standard as defined by the following equation (Coplen 2011): δ13C, δ15N = Rsample/Rstandard − 1, where R = 13C/12C or 15N/14N. The standards for carbon and nitrogen are Belemnite (VPDB) and atmospheric nitrogen, respectively. The reference materials were sucrose (sucrose ANU) for δ13C and ammonium sulfate (IAEA-N1) for δ15N that are certified by International Atomic Energy Agency (Viena, Austria). An internal working standard (L-α-alanine, -20.57‰ in δ13C and 7.84‰ in δ15N) was run every 5–6 samples. Analytical precision was ±0.17‰ for δ13C and ±0.25‰ for δ15N.
Statistical analysis
Wilcoxon signed rank testing was performed for the comparison of the isotopic values of the cormorants between the muscle and the liver and between the defatted tissue and the untreated tissue, and for the comparison of the assumed isotopic values of the past diets between muscle-based and liver-based calculations. Mann–Whitney’s U-test was performed for the comparison of the isotopic values of the mass-release ayu between the sampling months. Isotopic differences in the cormorant tissues among years (2009–2011) were analyzed by Kruskal–Wallis test and the subsequent Dunn’s multiple comparison tests. Isotopic differences between different developmental stages and between sexes were also analyzed by Kruskal–Wallis test and the Dunn’s multiple comparison tests. All the statistical analyses were performed using Prism 6.0 h (GraphPad Software).
Results
Stomach contents of Great Cormorants
A total of 13 fish species, including 102 individuals and two prawn (Macrobrachium nipponense and digested Caridean species), were extracted from 19 cormorants culled in the colony (Table 2). The detailed information about the cormorants having contained the prey in the stomach (Table S1) and the respective prey species (Table S2) is shown in Electronic Supplementary Material (ESM). Ayu (Plecoglossus altivelis altivelis), the most important mass-release fish in the watershed, was found in the stomachs of seven cormorants with relatively high occurrence frequency (9.6%). The occurrence of Japanese dace (T. hakonensis) in the cormorant stomachs was also relatively frequent with a frequency of 8.2% (a total of six cormorants). We also found two mass-release species, amago trout (Oncorhynchus masou ishikawae) in three cormorants and rainbow trout (Oncorhynchus mykiss) in two cormorants.
The dace (T. hakonensis) was conspicuous in total number of individuals extracted from the cormorant stomachs (n = 19) and the total wet weight (348.3 g) (Table 2). The rainbow trout (O. mykiss) was also abundant in the total weight (289.6 g), but not in total number of the extracted individuals (n = 3). By contrast, the ayu (P. altivelis altivelis) totaled 95.4 g only in weight, but was relatively high in the occurrence frequency (9.6%) and total number of the extracted individuals (n = 15). The amago trout (O. masou ishikawae) (145.5 g) was also not much in weight in disproportion to the catch of relatively many individuals (n = 12).
The body length (standard length) of the stomach-content fish ranged from 32.6 mm in Japanese fluvial sculpin (Cottus reinii) to 350.0 mm in rainbow trout (O. mykiss). There were of course damaged fish that could not be measured for standard length. The sizes of the ayu (P. altivelis altivelis) and the dace (T. hakonensis) extracted from the stomachs were similar, with 92.8–139.3 mm in the ayu and 86.2–171.2 mm in the dace.
Distributions of fish in the Kano River watershed
A total of 30 fish species including 4980 individuals were collected by means of throw nets in the Kano River watershed from July 2008 to December 2009 (Table 3, Appendices 1–5 in the ESM). Two cyprinid juveniles could not be identified. The fish consisted mainly of Cyprinids (n = 3803) and Salmonids (n = 496), occupying 76.4% and 10.0% of all the 4980 individuals, respectively. The result in our research is largely consistent with the result of the fish fauna research in the 1970 s by Itai (1982).
Downstream fatminnow (Rhynchocypris lagowskii) were most frequently collected in the throw-net sampling, mostly distributed in the tributaries of the entire watershed (20 stations). In combination with the result in the supplementary sampling, the downstream fatminnow were collected at 48 stations (Fig. 2a). The total catch of this species in the throw-net sampling was the third most abundant with 717 individuals.
Distributions of a Downstream fatminnow Rhynchocypris lagowskii (after Takai et al. 2012), b Japanese dace Tribolodon hakonensis, c Amago trout Oncorhynchus masou ishikawae, d Pale chub Zacco platypus, e Ayu Plecoglossus altivelis altivelis, f Amur goby Rhinogobius nagoyae, g Japanese fluvial sculpin Cotus reinii, and h Rainbow trout Oncorhynchus mykiss. Symbols: solid circles, collected; open circles, not collected
The dace (T. hakonensis) was also collected at many stations (17 stations) in the throw-net sampling, with the most abundant catch of 1810 individuals (Table 3). The dace was ubiquitously caught in both the main stream and the tributaries, with the catch at 23 stations in the total sampling (Fig. 2b).
The amago trout (O. masou ishikawae) (12 stations), pale chub (Zacco platypus) (11 stations), and the ayu (P. altivelis altivelis) (10 stations) were also collected with throw nets at relatively many stations. The amago trout were mostly collected in the upper reaches of the mainstream and the tributaries within the Izu Peninsula (Fig. 2c). The total catch of this species in the throw-net sampling was also abundant, specifically fourth in abundance (n = 428, Table 3). The pale chub and the ayu were mostly collected in the mainstream and the northeastern tributaries (Fig. 2d, e), becoming the second most (812 individuals) and the fifth most (217 individuals) abundant catch in the throw-net sampling (Table 3), respectively.
Amur goby (Rhinogobius nagoyae) and Japanese fluvial sculpin (C. reinii), extracted from the stomachs of 2–3 cormorants (Table 2), were mostly collected in the mainstream (Fig. 2f, g). The rainbow trout (O. mykiss), mass-release species, were collected in the northern and northeastern tributaries only (Fig. 2h).
Stable isotope ratios of Great Cormorants
Stable isotope ratios of the cormorant tissues ranged from − 23.0 to − 14.7‰ in δ13C and 8.9–18.4‰ in δ15N for defatted muscles and − 23.9 to − 13.9‰ in δ13C and 9.8–19.4‰ in δ15N for defatted livers (Table 4, Fig. 3a). The isotopic values of the livers were significantly higher than the values of the muscles for both δ13C and δ15N (Wilcoxon signed rank test; δ13C, P < 0.0001; δ15N, P < 0.0001).
δ13C–δ15N map for a values analyzed for muscles (solid squares) and livers (open circles) of Great Cormorants Phalacrocorax carbo and b past diet values calculated on the basis of muscles (solid squares) and livers (open circles). According to the enrichment factors experimentally examined by Mizutani et al. (1991), past diet values of the cormorants are calculated by subtracting 2.1‰ in δ13C and 2.4‰ in δ15N from the cormorant values for muscle-based calculation and 1.3‰ in δ13C and 2.3‰ in δ15N for liver-based calculation
There are not clear isotopic differences in the cormorant tissues among years (2009–2011) in either muscles or livers as shown in Fig. S1 (ESM). We performed Kruskal–Wallis tests and the subsequent Dunn’s multiple comparison tests for four categories, δ13C and δ15N of muscles and livers, respectively. In the result, there was no significant difference among the 3 years in every category (Kruskal–Wallis tests, P > 0.05), and there was no significant difference in every combination of the years in every category (Dunn’s multiple comparison tests, P > 0.05).
Furthermore, clear differences were neither found between different developmental stages nor between sexes (Figs. S2, S3 in the ESM). We performed Kruskal–Wallis tests and the subsequent Dunn’s multiple comparison tests for four categories, δ13C and δ15N of muscles and livers, respectively. In the result, there was no significant difference among four groups, the adults and the young of females and males, in every category (Kruskal–Wallis tests, P > 0.05), and there was no significant difference in every combination of the groups in every category (Dunn’s multiple comparison tests, P > 0.05).
Mizutani et al. (1991) experimentally demonstrated that the diet of Great Cormorants is 2.1‰ lower in δ13C and 2.4‰ lower in δ15N than the muscle of the cormorant and 1.3‰ lower in δ13C and 2.3‰ lower in δ15N than the liver of the cormorants. Subtracting these values from the values of the cormorant tissues, the assumed isotopic values of the past diets were calculated to be − 25.1 to − 16.8‰ in δ13C and 6.5–16.0‰ in δ15N for muscle-base calculation, and − 25.2 to − 15.2‰ in δ13C and 7.5–17.1‰ in δ15N for liver-base calculation (Fig. 3b). The liver-based dietary values were significantly higher than the muscle-based dietary values in both δ13C and δ15N (Wilcoxon signed rank test; δ13C, P < 0.0001; δ15N, P < 0.0001).
According to the statistical comparison between the defatted tissue and the untreated tissue of the identical cormorant (n = 30), both δ13C and δ15N were significantly higher in the defatted tissue than in the untreated tissue (Table 5, Wilcoxon signed rank test, P < 0.0001 in both δ13C and δ15N for the muscle and the liver respectively). The differences in the averages of the δ13C were 1.1 ± 0.4‰ for the muscle and 1.2 ± 0.5‰ for the liver, slightly larger than the differences of the δ15N with 0.3 ± 0.2‰ for the muscle and 0.2 ± 0.2‰ for the liver.
Stable isotope ratios of ayu
The stable isotope ratios of the ayu (P. altivelis altivelis) collected with throw nets ranged − 28.5 to − 12.2‰ in δ13C and 4.4–12.6‰ in δ15N (69.4–152.8 mm body length, Table 6, Fig. 4a). The isotopic distribution of the ayu was markedly different between the northeastern tributary (H1 and H2) and the mainstream (C1–C6) (Fig. 5). The ayu from the northeastern tributary were characterized by low δ13C values of − 28.5 to − 19.8‰, whereas the ayu from the mainstream was characterized by high δ13C values of − 19.8 to − 12.2‰ and low δ15N values of 4.4–10.8‰, except for five individuals from C1 and an individual from C3. The ayu collected at E2, a tributary station neighboring on the mainstream, overlapped in δ13C and δ15N with the ayu from the mainstream.
δ13C–δ15N map for fishes collected in the Kano River watershed (open rectangles) and past diet values calculated on the basis of muscles (solid squares) and livers (open circles) of Great Cormorants Phalacrocorax carbo. The fishes are shown as mean ± SD. a Ayu Plecoglossus altivelis altivelis. Symbols: Cl, the lower reaches of the mainstream (Stations C1–C2); Cu, the upper and middle reaches of the mainstream (C3–C6); E, the eastern tributary (E2); Ne, the northeastern tributary (H1–H2); R1, mass-release ayu (15 March 2013); R2, mass-release ayu (16 April 2013). b Japanese dace Tribolodon hakonensis. Symbols: C, the main stream (C1–C6); E, the eastern tributary (E1–E2); W, the western tributary (W3); N, the northern tributary (N2–N5); Ne, the northeastern tributary (H1–H3); Nw, the northwestern tributary (N1)
δ13C–δ15N map for ayu Plecoglossus altivelis altivelis extracted from stomachs of Great Cormorants Phalacrocorax carbo and collected by throw-net sampling in the Kano River watershed: Stations (Stns.) C1–C6 in the mainstream, Station (Stn.) E2 in the eastern tributary, and Stns. H1–H2 in the northeastern tributary
The stable isotope ratios of the ayu extracted from the stomachs of the culled cormorants ranged from − 18.6 to − 10.9‰ in δ13C and 6.8–14.3‰ in δ15N (Table 7). In the δ13C–δ15N map, the distribution of the stomach-content ayu partly overlapped with the distribution of the ayu collected with throw nets in the mainstream (C2–C6) (Fig. 5).
The stable isotope ratios of the mass-release ayu ranged from − 18.1 to − 17.3‰ in δ13C and 11.9–13.2‰ in δ15N for the individuals released on 15 March 2013 and ranged from − 17.4 to − 17.0‰ in δ13C and 13.8–14.2‰ in δ15N for the individuals released on 16 April 2013 (Table 8, Fig. 5). The δ13C averaged − 17.7 ± 0.2‰ in March and − 17.2 ± 0.1‰ in April, 1.2–1.7‰ higher than the δ13C value of − 18.9‰ for formula feed supplied to the rearing pond. There was a significant difference between March and April (Mann–Whitney’s U-test, P < 0.0001). The averages of δ15N in March (12.5 ± 0.5‰) and April (14.0 ± 0.2‰) were also higher than the δ15N value of 12.1‰ for the formula feed. In the δ15N, the difference between the formula feed and the mass-release ayu (averages) was larger in April (1.9‰) than the difference in March (0.4‰). According to Mann–Whitney’s U-test, a significant difference was found for the δ15N of the ayu between March and April (P < 0.0001).
Stable isotope ratios of Japanese dace
The stable isotope ratios of the dace (T. hakonensis), which was a conspicuous species in the stomach contents of the culled cormorants, were analyzed for the individuals collected with throw nets in the Kano River watershed. The isotopic values of this species ranged from − 25.4 to − 14.3‰ in δ13C and 4.3–13.6‰ in δ15N (40.5–158.9 mm body length, Table 9). There were marked differences among the sampling stations.
The isotopic variety in the dace from the watershed was mainly characterized by local differences among tributary stations, with low δ13C values (− 25.4 to − 23.8‰) for Stn. E1, high δ13C values (− 19.6 to − 14.3‰) for N1, low δ15N values (4.3–5.5‰) for W3, and high δ15N values (12.0–13.6‰) for N2–N5 (Fig. 4b). On the other hand, the stable isotope ratios of the dace extracted from the stomachs of the culled cormorants ranged from − 22.6 to − 14.2‰ in δ13C and 6.6–13.1‰ in δ15N (Table 7). In the δ13C–δ15N map, the distribution of the stomach-content dace overlapped with the distribution of the dace collected by the throw-net sampling (Fig. 6). Most of the stomach-content dace overlapped with the dace from the mainstream (C1–C6) and its neighboring tributary station (E2), the northern tributary (N1–N5), and the northeastern tributary (H1–H3).
δ13C–δ15N map for Japanese dace Tribolodon hakonensis extracted from stomachs of Great Cormorants Phalacrocorax carbo and collected by the throw-net sampling in the Kano River watershed: Stations (Stns.) C1–C6 in the mainstream, Stns. E1–E2 in the eastern tributary, Station (Stn.) W3 in the western tributary, Stns. N1–N5 in the northern tributary, and Stns. H1–H3 in the northeastern tributary. The isotopic values of a dace collected at the river mouth station of the Inohzawa River are also shown
Only two dace extracted from a cormorant showed a peculiar isotopic distribution (− 16.1 to − 15.2‰ in δ13C and 12.5–13.1‰ in δ15N), separating from the dace collected in the Kano River watershed (Fig. 6). This isotopic distribution is close to the isotopic values of Japanese horse mackerel (T. japonicus) collected in the river mouth of the Kano River, and the dace collected in the river mouth of the Inohzawa River (Table 9).
Discussion
Food supply from mass-release ayu
Ayu (P. altivelis altivelis) was most frequently found in stomachs of culled cormorants with an occurrence frequency of 9.6%, although the catch of ayu in the year-round fish fauna research totaled only 217 individuals (Tables 2, 3). This apparent inconsistency, frequent preying and relatively poor catch in the watershed, can be explained by the release of numerous ayu in spring and summer. The total of the ayu released to the Kano River approximated to 370,000 individuals in 2009, 330,000 in 2010, and 310,000 in 2011 (Table 1). Therefore, the Kano River watershed had temporarily been crowded with the mass-release ayu in the releasing season. We did not perform the throw-net sampling in the mainstream during the main releasing season, late March to early July, to avoid causing damage to the population of the released ayu. Therefore, the result of the throw-net sampling should not strongly reflect the temporal abundance of the released ayu. The relatively frequent occurrence of ayu in the stomach content analysis suggests that the cormorants had utilized the mass-release ayu as a substantial food source.
This substantial feeding on the mass-release ayu in the season was supported by the stable isotope analysis. The isotopic values of some ayu individuals extracted from the stomachs of the culled cormorants were similar to the isotopic values of ayu caught in the watershed (Fig. 5). It is likely that these stomach-content individuals more depleted in 15N had stayed in the watershed and fed on natural diets there over 1 month after their migration from the sea or releasing as a part of mass-release ayu. However, the other stomach-content ayu showed peculiarly high δ15N values, clearly distinct from the δ15N of the ayu caught in the watershed, and overlapped with the values of mass-release ayu. This result demonstrates that the cormorants preyed on the mass-release ayu enriched in 15N in the season.
The distinct isotopic distribution peculiar to the mass-release ayu is well consistent with isotopic values of formula feed used in the seed production facility. Both δ13C and δ15N of the mass-release ayu were higher than the values of the formula feed (Fig. 5). Particularly, the averages of the ayu released on April 16 were significantly higher than the averages of the ayu on March 15, with differences of 0.5‰ in δ13C and 1.5‰ in δ15N. The ayu released on March 15 had been reared in the facility for about a week, while the ayu released on April 16 had been reared for about a month (The Kano River Fisheries Cooperative Association, personal communications). The feeding of the formula feed enriched in 15N during a longer period likely resulted in the higher δ15N values of the ayu released on April 16. This result suggests that the isotopic peculiarity of the mass-release ayu was derived from the isotopic distribution of the formula feed.
The isotopic values of past diets inferred by the isotope analysis of livers of the culled cormorants were significantly higher than the past dietary values inferred by the analysis of the cormorant muscles, and were closer to the values of the mass-release ayu (Fig. 4a). The isotopic values of muscles and livers approximately reflect the feeding behavior during the past month and the past week, respectively (Hobson and Clark 1992). Therefore, the enrichment of 13C and 15N in livers relative to muscles means that recent diets of the cormorants were more enriched in 13C and 15N than previous diets. This result suggests that the food supply from the mass-release ayu had increased in the breeding season.
These results suggest that there was an anthropogenic food chain from the formula feed of the mass-release ayu to the cormorants through the prey-predator relationship between the cormorants and the ayu. The cormorants in the Kano River basin had mainly nested from April to July in 2010 and 2011 (Kawabe et al. 2013). This nesting season almost overlaps with the releasing season of the ayu (Table 1). We infer that numerous ayu released in the watershed largely supported the breeding of the cormorants in the season.
Food supply from Japanese dace
Japanese dace (T. hakonensis) was also frequently found in stomachs of culled cormorants with an occurrence frequency of 8.2%, and was the most abundant in total number of individuals (n = 19) and the total weight (348.3 g) in the cormorant stomachs (Table 2). Furthermore, the stable isotopic distribution of the dace extracted from the cormorant stomachs overlapped with the values of the dace collected by throw-net sampling in the mainstream and the tributaries of the Kano River watershed (Fig. 6). These results suggest that the cormorants had frequently and abundantly preyed on the dace in the breeding season. In the fish fauna research, the dace was most abundantly collected in the Kano River watershed with a total of 1810 individuals at 17 stations (Table 3, Fig. 2b). The dace is likely easy prey for the cormorants, because of its abundance and broad distribution in the watershed.
Two Japanese dace individuals extracted from a culled cormorant showed peculiar isotopic distribution, with high values in both δ13C (− 16.1 to − 15.2‰) and δ15N (12.5–13.1‰). This isotopic distribution is close to the isotopic values of Japanese horse mackerel (T. japonicus) collected in the river mouth of the Kano River (Table 9). Additionally, we had collected a dace similarly enriched in both 13C and 15N from the river mouth of the Inohzawa River, the southeastern area of the peninsula (Fig. 6). Japanese dace has two forms of life style within the species: the landlocked form inhabiting the upper and middle reaches of the river and the diadromous form inhabiting the estuarine zone of the river mouth (Hosoya 2000). The δ13C and δ15N of the diadromous form would increase through feeding on marine organisms, since both δ13C and δ15N of marine organisms are generally higher than those of freshwater organisms (Takai 2005). The year-round census in 2010 and 2011 by Kawabe et al. (2013) showed that some cormorants had stayed in the river mouth of the Kano River during the daytime. Thus, we infer that the two stomach-content dace enriched in both 13C and 15N were diadromous fish, preyed upon by the cormorant in the river mouth area.
Here we add that this explanation for the dace does not apply to the above-mentioned enrichment in 15N of the stomach-content ayu. The ayu also spends its life in the coastal area and the river mouth at the larval and juvenile stages, but the juvenile ayu migrate upstream at the body size of about 50 mm (Otake 2006). The body size of the ayu extracted from the cormorant stomachs ranged from 92.8 to 139.3 mm, and therefore the stomach-content ayu individuals enriched in 13C and 15N were unlikely dwellers in the river mouth.
Stable isotopic distribution of the past diet values inferred by the isotope analysis of the muscles of the culled cormorants was well consistent with the distribution of the dace collected by the throw-net sampling in the watershed, but the distribution of the past diet inferred by the analysis of the cormorant livers slightly deviated from the distribution of the dace in the watershed (Fig. 4b). This result suggests that the food supply from the dace had decreased in the breeding season, since the isotopic turnover rate is faster in livers. The dace was abundantly collected in every season in the watershed, suggesting that the dace is abundant in the watershed throughout a year. The dace is likely the most important food source for the cormorants before the releasing season of the ayu.
Food supply from other fishes
Occurrence frequency of pale chub (Z. platypus) and downstream fatminnow (R. lagowskii) in stomachs of culled cormorants was low, despite being the second and the third most abundant catch in the throw-net sampling (Tables 2, 3). The pale chub generally prefer the shallows in rivers (Mori and Nagoshi 1989), and downstream fatminnow mainly inhabit narrow mountain streams (Takai et al. 2012). Therefore, these two species are likely more difficult prey for the cormorants, which have the habit of diving to forage in the deep pools of rivers.
Stomach contents of culled cormorants included 14 Japanese fluvial sculpin (C. reinii) (Table 2), although the cormorants in central Japan have not been reported to prey on this species (Kameda et al. 2002, Tsuchiya et al. 2013). Additionally, eight individuals of Amur goby (R. nagoyae and R. mizunoi) were found in the stomach of four culled cormorants (Table 2). Both the sculpin and the Amur goby are small demersal species settling on bottoms in the middle or lower reaches of rivers (Miyadi et al. 1976, Goto 1989). The cormorants in the Kano River basin likely prey on the bottom inhabitants as well as nektonic species such as ayu and dace, suggesting that the food source for the cormorants is somewhat diverse.
Foraging behavior of the Great Cormorants
The 13 species of prey fish found in the cormorant stomachs were distributed in varied areas of the Kano River watershed. For example, the Amur goby (R. nagoyae) was mostly collected in the mainstream flowing across the Izu Peninsula (Fig. 2f), whereas the amago trout (O. masou ishikawae) was collected in the upper reaches of the mainstream and tributaries only (Fig. 2c), and the rainbow trout (O. mykiss) was collected in the northern and northeastern tributaries only (Fig. 2h). These results indicate that the Kano River population of the cormorants had foraged around the entire area of the watershed.
Tsuchiya et al. (2013) showed that the cormorants breeding in coastal colonies (≤ 15 km from the seashore) had mainly preyed on seawater fishes, gray mullet Mugil cephalus cephalus and dotted gizzard shad Konosirus punctatus, and the cormorants in inland colonies (> 15 km from the seashore) had preyed various kinds of fish species, based on a stomach content analysis of the cormorants in the Chubu region, Japan. In our study area, the colony was located near the seashore with 1 km distance (Fig. 1), but only a single cormorant contained the gray mullet in the stomach and the gizzard shad was not extracted from the cormorant stomachs (Table 2). It appears that the diversity of the prey species in the Kano River basin is similar to the dietary pattern in the inland colony in Tsuchiya et al. (2013). There are likely regional differences in the relationship between the food habit of the breeding cormorants and the distance from the sea to the colony.
The census of the cormorants in the Kano River watershed by Kawabe et al. (2013) found that main part of the feeding ground for the cormorants was located in the middle and lower reaches of the mainstream. This distribution of the feeding ground is well consistent with the distributions of the ayu and the dace. These two species were abundantly collected in the middle and lower reaches of the mainstream (Table 3). Hino and Ishida (2012) showed that the distance from the roost or the colony of the cormorants to the feeding ground was 2–11 km on average by a GPS-Argos tracking experiment in the Tokai region, Japan. The middle and lower reaches of the Kano River mainstream are mostly located within 11 km from the colony (Fig. 1), and therefore the fish in the reaches are likely available for the cormorants. Thus, we inferred that the cormorants had mainly foraged for these fishes in the areas.
As mentioned above, the analyses of stomach content compositions and stable isotope ratios suggest that the ayu becomes relatively important as a food source for the cormorants during the mass-release season of the ayu. The mass release throughout the middle and lower reaches gives rise to aggregation patches of the ayu everywhere, and thus the cormorants can search for prey in a short time and capture them easily. Accordingly, the cormorants can minimize the cost of foraging and capturing if they selectively forage the mass-release ayu. Kumada et al. (2014) reported that the size of roosts or colonies of the cormorants increased from March to July in the Kanto region, Japan, when mass-release ayu was abundantly released near the roosts or the colonies, and suggested that it may be caused by the enhancement of the reproductive success or its immigration from other areas. In riverine ecosystems where a large number of ayu are released, the selective foraging of the mass-release ayu is likely the best foraging strategy for the cormorants during the releasing season.
The results of the stable isotope analysis also suggest that a cormorant had peculiarly utilized more distant feeding ground. The cormorant culled in May 2010 showed especially high δ15N values for both the muscle (18.4‰) and the liver (19.4‰) (Fig. 3a). The δ13C values of this individual were also high for both the muscle (− 14.7‰) and the liver (− 13.9‰). Based on isotopic enrichment factors for the cormorant (Mizutani et al., 1991), the prey values of this individual were calculated to be − 16.8 to − 15.2‰ in δ13C and 16.0–17.1‰ in δ15N (Fig. 3b). The inferred δ15N values of 16.0–17.1‰ for the past prey were clearly located off the δ15N distributions of the ayu and the dace (Fig. 4). It was considered that this cormorant had not utilized the Kano River watershed as a main feeding ground and had fed on 15N-enriched prey in a distant place.
Such high isotopic values of small fish were reported for marine species, Japanese anchovy Engraulis japonicus, collected in the eastern part of the Sagami Bay, with a maximum of − 14.2‰ in δ13C and 17.8‰ in δ15N (Tanaka et al. 2008). Similarly high isotopic values were reported for marine fish and invertebrates in another eutrophicated area (Takai et al. 2002). It is well known that the home range of the cormorants is broad in some cases. Satellite tracking experiments found that a cormorant flew between Lake Kasumigaura (to the northeast of the Tokyo Bay) and the southern Chubu region, which is over 300 km, twice over 3 months (Ministry of the Environment 2004). Thus, the cormorant is capable of migrating between the Kano River basin and the eastern part of the Sagami Bay during a short period. There are many colonies and roosts of the cormorants in the region around the northern Tokyo Bay (Ministry of the Environment 2004). It is inferred that the cormorant with especially high δ15N values had utilized distant eutrophicated sea area as a main feeding ground. This individual might be an immigrant that had flown to the colony during the breeding season, in other words, mass-release season of ayu.
Conclusion
The results of this study suggest that the cormorants in the Kano River basin utilize Japanese dace as a main food source before the breeding season, and in the breeding season substantially utilize mass-release ayu as well. This suggests that the breeding of the cormorants is largely supported by the seasonal occurrence of an anthropogenic food chain from the formula feed in the seed production to the cormorants through their feeding on mass-release ayu. The total catch of the ayu in Japan had monotonically increased from the 1950 s to the early 1990 s, largely contributed by the progress of the seed production system (Uchida 2010). It is probable that the seasonal trophic link between the cormorants and the mass-release ayu in the breeding season had enhanced reproductive success of the cormorants throughout the country and consequently contributed to the recovery of the cormorant population.
References
Bearhop S, Thompson DR, Waldron S, Russell IC, Alexander G, Furness RW (1999) Stable isotopes indicate the extent of freshwater feeding by cormorants Phalacrocorax carbo shot at inland fisheries in England. J Appl Ecol 36:75–84
Coplen TB (2011) Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results. Rapid Commun Mass Spectorom 25:2538–2560
Deniro MJ, Epstein S (1978) Influence of diet on distribution of carbon isotopes in animals. Geochim Cosmochim Acta 42:495–506
Deniro MJ, Epstein S (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta 45:341–351
Doucette JL, Bjorn W, Somers CM (2011) Cormorant–fisheries conflicts: stable isotopes reveal a consistent niche for avian piscivores in diverse food webs. Ecol Appl 21:2987–3001
Fisheries Agency (2003) The report for the development project of management of inland water ecosystems: preventive measure for the feeding damages by Great Cormorant and other animals. Fisheries Agency, Tokyo
Fry B (1988) Food web structure on Georges Bank from stable C, N, and S isotopic compositions. Limnol Oceanogr 33:1182–1190
Fukuda M, Narusue M, Kato N (2002) Changes in the distribution and abundance of the Great Cormorant Phalacrocorax carbo in Japan. Jpn J Ornithol 51:4–11
Goto A (1989) Cottus pollux. In: Kawanabe H, Mizuno N, Hosoya K (eds) Freshwater fishes of Japan. YAMA-KEI Publishers, Tokyo, pp 666–667
Hino T, Ishida A (2012) Home ranges and seasonal movements of Great Cormorants Phalacrocorax carbo in the Tokai area, based on GPS-Argos tracking. Jpn J Ornithol 61:17–28
Hobson KA, Clark RG (1992) Assessing avian diets using stable isotopes I: turnover of 13C in tissues. Condor 94:181–188
Hosoya K (2000) Cyprinidae. In: Nakabo T (ed) Fishes of Japan with pictorial keys to the species, 2nd edn. Tokai University Press, Tokyo, pp 253–271
Iguchi K, Tsuboi J, Tsuruta T, Kiryu T (2008) Foraging habits of great cormorant in relation to released ayu stocks as a food source. Aquacult Sci 56:415–422
Itai T (1982) Freshwater fish in Shizuoka Prefecture (Shizuokaken no tansuigyorui). Daiichihoki, Tokyo
Kameda K, Matsubara T, Mizutani H, Yamada Y (2002) Diet and foraging site selection of the Great Cormorant in Japan. Jpn J Ornithol 51:12–28
Kawabe K, Kumon Y, Togura K, Takai N (2013) Habitat use of the Great Cormorant Phalacrocorax carbo in the Kano River watershed, the Izu Peninsula region, central Japan. Strix 29:29–43
Kumada N, Fujioka M, Motoyama Y (2014) Does the mass stocking of fish affect the size and distribution of Great Cormorant roosts and colonies? Jpn J Ornithol 63:23–32
Levine S (1980) Several measures of trophic structure applicable to complex food webs. J Theor Biol 83:195–207
Minagawa M, Wada E (1984) Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochim Cosmochim Acta 48:1135–1140
Ministry of Agriculture, Forestry and Fisheries (2010) The 2008 fishery census of Japan. Ministry of Agriculture, Forestry and Fisheries, Tokyo
Ministry of the Environment (2004) The technical manual for the specified wildlife conservation and management plan (Great Cormorant). Ministry of the Environment, Tokyo
Miyadi D, Kawanabe H, Mizuno N (1976) Colored illustrations of the freshwater fishes of Japan. Hoikusha Publishing, Osaka
Mizutani H, Kabaya Y, Wada E (1991) Nitrogen and carbon isotope compositions relate linearly in cormorant tissues and its diet. Isotopenpraxis 27:166–168
Mori S, Nagoshi M (1989) Zacco platypus. In: Kawanabe H, Mizuno N, Hosoya K (eds) Freshwater fishes of Japan. YAMA-KEI Publishers, Tokyo, pp 244–249
Nakabo T (2000) Fishes of Japan with pictorial keys to the species, 2nd edn. Tokai University Press, Tokyo
National Federation of Inlandwater Fisheries Cooperatives (2004) The research of the feeding damage to fisheries species by Great Cormorant. http://www.naisuimen.or.jp/jigyou/kawau/kawau16.pdf. Accessed 29 June 2017
Natsumeda T, Sakano H, Tsuruta T, Kameda K, Iguchi K (2015) Immigration of the common cormorant Phalacrocorax carbo hanedae into inland areas of the northern part of Nagano Prefecture, eastern Japan, inferred from stable isotopes of carbon, nitrogen and sulphur. Fish Sci 81:131–137
Nishida M (1989) Plecoglossus altivelis altivelis. In: Kawanabe H, Mizuno N, Hosoya K (eds) Freshwater fishes of Japan. YAMA-KEI Publishers, Tokyo, pp 66–79
Otake T (2006) Early life history of ayu in coastal waters. Bull Fish Res Agency Suppl 5:179–185
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718
Rau GH, Mearns AJ, Young DR, Olson RJ, Schafer HA, Kaplan IR (1983) Animal 13C/12C correlates with trophic level in pelagic food webs. Ecology 64:1314–1318
Simpson K, Day N (2004) Field guide to the birds of Australia. Christopher Helm Publishers, London
Takahashi T, Kameda K, Kawamura M, Nakajima T (2006) Food habits of great cormorant Phalacrocorax carbo hanedae at Lake Biwa, Japan, with special reference to ayu Plecoglossus altivelis altivelis. Fish Sci 72:477–484
Takai N (2005) Carbon and nitrogen stable isotope ratios indicative of organic matter flow in the ecosystem of the Seto Inland Sea, Japan. Jpn J Ecol 55:269–285
Takai N, Mishima Y, Yorozu A, Hoshika A (2002) Carbon sources for demersal fish in the western Seto Inland Sea, Japan, examined by δ13C and δ15N analyses. Limnol Oceanogr 47:730–741
Takai N, Kato A, Uekusa M, Kimura Y, Dairiki K, Itoi S, Sugita H, Yoshihara K (2011) Longitudinal distribution of fishes in the Inohzawa River watershed, southern Izu Peninsula, Japan. Jpn J Ichthyol 58:13–25
Takai N, Abiko Y, Tsukamoto H, Miura A, Yuasa K, Itoi S, Nakai S, Sugita H, Yoshihara K (2012) Species identication of upstream fatminnow Rhynchocypris oxycephalus and downstream fatminnow Rhynchocypris lagowskii, based on PCR-RFLP of mitochondrial DNA. Ichthyol Res 59:156–163
Tanaka H, Takasuka A, Aoki I, Ohshimo S (2008) Geographical variations in the trophic ecology of Japanese anchovy, Engraulis japonicus, inferred from carbon and nitrogen stable isotope ratios. Mar Biol 154:557–568
Tsuchiya K, Kazama K, Inoue Y, Fujii H, Niizuma Y (2013) Inter-colonial and annual differences in food habits of Great Cormorants rearing chicks in the Chubu area of Japan. Jpn J Ornithol 62:57–63
Uchida K (2010) Fisheries management for stock enhancement in ayu. Nippon Suisan Gakkaishi 76:416
Wada E, Terazaki M, Kabaya Y, Nemoto T (1987) 15N and 13C abundances in the Antarctic Ocean with emphasis on the biogeochemical structure of the food web. Deep Sea Res 34:829–841
Acknowledgements
We thank the Kano River Fisheries Cooperative Association and the Tagata Hunting Club for their cooperation with the collection of fish and cormorants. We are also grateful to M. Sato, E. Yagino, Y. Suzuki, Y. Kumon, S. Sugiyama, I. Tachibana, M. Kamataki, Y. Takanashi, H. Kojima, H. Cho, and M. Nakata for their cooperation with the sampling and the dissection. This study was financially supported by a Grant-in Aid for Scientific Research from Ministry of Education, Culture, Sports, Science and Technology (no. 22580210).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Takai, N., Kawabe, K., Togura, K. et al. The seasonal trophic link between Great Cormorant Phalacrocorax carbo and ayu Plecoglossus altivelis altivelis reared for mass release. Ecol Res 33, 935–948 (2018). https://doi.org/10.1007/s11284-018-1610-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-018-1610-4