Abstract
Silicon (Si) has various biogeochemical functions, such as regulating soil formation and species composition, not only in terrestrial ecosystems but also in aquatic ones. Bamboo stands accumulate large quantities of amorphous Si. Evaluating Si dynamics in moso bamboo (Phyllostachys pubescens) forests, which are currently spreading through eastern Asia, is important in understanding their biogeochemical function as a supply source of phytoliths. We conducted a study on the organic accumulation and biological cycle of Si in three P. pubescens stands in central Japan with different site characteristics. The amounts of Si accumulation aboveground and underground were 200–360 and 180–460 kg/ha, respectively. These values indicate that Si accumulation underground was comparable to that aboveground. Silicon supply to the forest floor through litterfall was 77–330 kg/ha/year corresponding to 165–706 kg/ha/year as phytoliths (SiO2), and 72–88 % was supplied as leaf litter. These results showed that a huge biogenic Si pool derived from bamboo plants exists in the floor of bamboo forests. Furthermore, we estimated the Si turnover time in P. pubescens forests as being 1.3–12.2 years, although this variation may depend on forestry conditions such as soil water content or stem density.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Silicon (Si) is the second-most abundant element after oxygen in the Earth’s crust (28.8 %), where it mostly exists as silicates, which comprise 90 % of all crust minerals (Wollast and Mackenzie 1983). The geochemical weathering of silicates is an important process in the carbon cycle and contributes to regulating atmospheric CO2 by reacting with carbonic acid (Berner 1995; Kump et al. 2000; Street-Perrott and Barker 2008; Song et al. 2011, 2012b), by occluding carbon within phytoliths, the silicified features that deposit within plant tissues (Parr et al. 2010; Song et al. 2013; Huang et al. 2014), and via oceanic diatom growth, which is sustained by Si transport from terrestrial environments to coastal zones (Raven and Falkowski 1999; Tréguer and Pondaven 2000; Rabosky and Sorhannus 2009).
Silicon pools in global ecosystems can be subdivided into mineralogical pools, such as primary minerals, secondary crystalline minerals, and non-crystalline (amorphous) phases (Drees et al. 1989; Matichenkov and Snyder 1996), and biogenic pools. These biogenic pools include phytogenic, microbial, and protozoic forms of Si (Struyf et al. 2009). Some higher plants use Si in the cell wall of their sustentacular and protective tissues to defend themselves from various biotic (e.g., disease) or abiotic (e.g., metal toxicity, salinity, drought, temperature extremes, and hydrodynamic forces from wind and water movement) environmental stresses (Raven 1983; Epstein 1999; Takahashi 2007; Schoelynck et al. 2010, 2012). Silicon exists in plant tissues as deposits of amorphous silica (SiO2·nH2O) called phytoliths or plant opals (Kondo 2010). After plant death, phytoliths contribute to the pool of amorphous Si in the upper soil layers and constitute an important component of soil systems (Struyf et al. 2009). Phytoliths released into the soil are physically and chemically weathered, after which dissolved Si can either circulate within forest ecosystems via plant re-uptake (Meunier et al. 1999; Conley 2002; Derry et al. 2005; Cornelis et al. 2010; Struyf and Conley 2012), be redeposited in soils as secondary silicate minerals such as allophanes or imogolites (Basile-Doelsch et al. 2005; Sommer et al. 2006; Gérard et al. 2008; Struyf et al. 2009), or exported to aquatic ecosystems (Conley 1997; Conley et al. 2008; Smis et al. 2011; Schoelynck et al. 2014). To link local vegetation-scale processes in terrestrial systems to larger-scale fluxes toward rivers and, ultimately, coastal zones, we must understand in plants their Si accumulation rates, Si physiology, and abundance of Si-accumulating species, as well as the eventual terrestrial–aquatic fluxes (Bartoli 1983; Conley 1997, 2002; Fulweiler and Nixon 2005; Conley et al. 2008; Schoelynck et al. 2014).
Although all terrestrially-grown plants contain Si in their tissues, the concentrations in shoots vary considerably among species, ranging from 1 to 100 mg Si/gdw (Epstein 1999; Ma and Takahashi 2002). These differences among species can be attributed to different abilities for Si uptake by roots, which can be active, passive, or rejective (Ma and Takahashi 2002). Plants with high Si content (>1.0 %) and high Si/Ca ratios (>1.0) are defined as Si accumulators with active uptake (Ma and Takahashi 2002). Gramineous plants are well-known as Si accumulators (Takahashi and Miyake 1976; Bakker et al. 1999). Therefore, grass-dominated vegetation types, such as bamboo forests, are considered to have large pools of biogenic amorphous Si in the upper soil layers (Struyf et al. 2009).
Bamboo includes over 70 genera with more than 1200 species and occupies more than 14 million ha worldwide (Dransfield and Widjaja 1995; Fu and Banik 1995). Almost 80 % of all bamboo species and forests are found in South and Southeast Asia, including China, India, and Myanmar (Kleinhenz and Midmore 2001), where bamboo is important not only as a bioresource but also for agriculture. Therefore, to use bamboo sustainably, bamboo forest management should emphasize nutrient cycling, and to that end, various studies have been conducted on nitrogen, phosphorus, and potassium in bamboo forests (Raghubanshi 1994; Mailly et al. 1997; Shanmughavel and Francis 1997; Li et al. 1998b; Embaye et al. 2005). Previous studies on Si behavior in bamboo plants have shown that Si concentrations in leaves and culms increase seasonally (Ueda et al. 1961; Nishida 1989) and that Si is deposited as plant opal in leaves, roots, and rhizomes (Motomura and Fujii 2000; Lux et al. 2003; Kondo 2010). Li et al. (2006) described the annual Si cycle of uptake, retention, and return in a moso bamboo (Phyllostachys pubescens) community in China. Bamboo also contributes to carbon bio-sequestration within silica phytoliths (Parr et al. 2010; Song et al. 2013; Huang et al. 2014), and harvesting substantially changes terrestrial Si export fluxes to rivers and oceans, because reconstitution of biogenic Si in litter fall is prevented (Vandevenne et al. 2012).
Quantitative values for Si cycles in bamboo stands should be interpreted with consideration of site location, stem density, bamboo size, and other factors, but there are few studies on Si cycling in bamboo forests. To increase our knowledge, the goals of this study were to evaluate Si dynamics in P. pubescens forests in central Japan and to understand their roles in the Si biogeochemical cycle of forest ecosystems.
Materials and methods
Site characteristics and physical and chemical properties of surface soil
This study was conducted in P. pubescens forests where stem density management or fertilization had not been conducted. The three sites were all in Aichi Prefecture, central Japan (Fig. 1): Kanpachi (35°07′N, 137°13′E, 110 m altitude), Seto (35°11′N, 137°07′E, 200 m altitude), and Noguchi (35°07′N, 137°15′E, 160 m altitude). The precise age of these forests has not been recorded. The annual mean temperature and precipitation at Toyota, the nearest AMeDAS (Automated Meteorological Data Acquisition System) observation site, is 14.8 °C and 1451.4 mm, respectively (Japan Meteorological Agency 2011).
We established a 15 m × 15 m quadrat in 2008 at each site. Mean diameter at breast height (DBH) and density of each bamboo stand were 8.2, 10.1, and 11.2 cm and 2660, 2400, and 4790 stems/ha at Kanpachi, Seto, and Noguchi, respectively (Table 1). The density of P. pubescens at each site was constant during the period of study, from June 2008 to December 2009. The bamboo stands in Kanpachi and Seto were located at the base of a mountain and near water flow. The stand in Noguchi was on a mountain side. The forests had sparse understory vegetation of evergreen and deciduous broad-leaved plants. Understory species comprised Cleyera japonica, Eurya japonica, Illicium anisatum, Osmanthus heterophyllus, Quercus glauca, Callicarpa mollis, and Wisteria sp. in Kanpachi and Aucuba japonica, Cleyera japonica, and Nandina domestica in Noguchi. Only sparse Cleyera japonica and Camellia sinensis were observed in Seto.
The underlying bedrock was amphibole granitite in all sites. Surface soil acidity (0–10 cm in depth) was measured as 4.5–4.8 pH (H2O), and similar concentrations (0.023–0.025 g/kg) of available SiO2 were found at each site (Table 2). Water content was lowest at Noguchi (0.31 kg/kg) (Umemura and Takenaka 2014).
Bamboo sample collection
We sampled current leaves from five bamboos of mean DBH in each quadrat once a month from June 2008 to May 2009 to observe seasonal changes in Si concentration. To investigate Si accumulation in each organ, one bamboo older than 4 years with mean DBH was harvested at each site in December 2009. Culms were cut at internodes at the culm base and at breast height, as well as 5, 10, and 15 m from the culm base (no samples were 15 m high in Kanpachi), and cut segments were cross-sectioned. Leaved branches located 0, 2, and 4 m from the lowest branch on each culm were also collected. The culms, branches, and leaves from each height were chemically analyzed.
Rhizomes were sampled from five randomly-placed subplots (30 cm soil depth × 50 cm × 50 cm) in each site in December 2009. Rhizomes were washed with tap water preliminarily to remove soil-derived silicon, and all developing roots were then cut off. Rhizome biomass at each site was calculated by averaging the biomass among the five subplots. Chemical analysis was performed using internode segments of five rhizomes selected randomly from the gathered samples.
To obtain fine root samples, five soil core samples were taken from each site using a liner core sampler (diameter 48.4 mm × depth 300 mm). All core samples were transported to a laboratory and kept frozen (−20 °C) until analysis was performed. In the laboratory, core samples were separated into six subsamples of 5 cm length. Bamboo roots collected from these subsamples were washed with tap water to remove contamination from silicon derived from soil. Roots were then classified as living or dead based on the definitions established by Vogt and Persson (1991). Living roots were separated into two diameter classes (d ≥ 2 mm, d < 2 mm).
Litterfall sample collection
Five litter traps 50 cm × 50 cm in size were randomly placed on the forest floor of each site in July 2008. Litter samples from each trap were collected once a month from August 2008 to July 2009. The litter was dried at 80 °C for 48 h and sorted carefully into bamboo leaves, leaf sheaths, and branches and into other organic matter.
Silicon analysis
For litter Si analysis, we used the litter samples collected in the month with the greatest litter supply for each organ and at each site. All samples of each bamboo organ were washed using an ultrasonic bath (<1 min), rinsed twice in deionized water, and dried at 80 °C for 48 h. To obtain homogeneous samples, culms, branches, and rhizomes were sliced and leaves and roots were ground using a mill.
We determined Si concentration using a combination method of wet digestion with nitric acid followed by gravimetric analysis for the insoluble portion and by inductively-coupled plasma atomic emission spectrometry (ICP-AES; IRIS ICARP, Jarrell Ash Nippon, Kyoto, Japan) for the soluble portion (Umemura and Takenaka 2010). The yield of Si obtained by this combination method was confirmed as 99.7 % using standard silica gel (Silica Gel 60, K640, SiO2·nH2O, >98 % pure grade, Katayama Chemical, Osaka, Japan).
Calculation of Si accumulation, supply, and turnover time
The Si concentration of each organ (culms, branches, and leaves) was determined by calculating the mean value of the Si concentrations at each height. Silicon accumulation in each bamboo organ per unit area was determined by multiplying the Si concentration in each organ by the corresponding biomass at each site. Culm, branch, and leaf biomass were calculated from the DBH of all bamboo individuals in each quadrat using P. pubescens-specific allometry equations derived from a previous study in western Japan (Okuda et al. 2007). For roots, the mean Si concentrations in each diameter class (d ≥ 2 mm, d < 2 mm) taken from the 5–10 cm depth were used to calculate total root Si concentration at 0–30 cm depth.
Annual Si supply via litterfall at each site was determined from the Si concentrations of each litter fraction and the corresponding litter biomass. Turnover time (year) of Si was calculated for each site by dividing total Si accumulation (above- and underground) by total Si supply to the forest floor via litterfall of bamboo organs.
Statistical analyses
Differences in leaf Si concentrations in each month in the three study sites were assessed by one-way ANOVA followed by Tukey’s HSD multiple comparison test (p < 0.05). Two-way ANOVA was used to test the significance of the main effects (organ and study site) and their interaction on Si concentrations of above- and underground organs. Tukey’s HSD test was performed to determine whether differences between individual factors were significant (p < 0.05). Significant differences in Si concentration in each litter organ were calculated by one-way ANOVA followed by Tukey’s HSD (p < 0.05) for each site. For these analyses, we used the free statistical software R version 2.15.1 (R Development Core Team 2012).
Results
Characteristics of Si concentration and biomass of each organ in P. pubescens
We found that the leaf Si concentrations increased from one spring to the next at all sites (Fig. 2), but the concentrations at Noguchi were significantly lower than those at the other sites in most months (p < 0.05). Silicon concentrations of aboveground P. pubescens organs were affected significantly by organ, study site, and their interactions, but there were no significant effects on Si concentrations in underground parts (Table 3; Fig. 3). Among aboveground organs, leaves possessed the highest Si concentrations (48–68 mg/g; Fig. 3a) and culms the lowest (1.2–3.5 mg/g). Among underground organs, fine roots (d < 2 mm) tended to contain more Si than coarse roots (d ≥ 2 mm) and rhizomes, although the differences were not significant (Fig. 3b). The root biomass distribution showed that fine roots were distributed at the upper soil layer (0–5 cm) but more coarse roots were found in the 5–10 cm layer (Fig. 4). The roots in the surface soil (0–10 cm) comprised 54–60 % of the total (0–30 cm) root mass.
Silicon concentrations in each organ of Phyllostachys pubescens. a Aboveground parts: culms, branches, and leaves. Error bars show SD among subsamples of each height of one bamboo stem for culms, branches, and leaves. b Underground parts: rhizomes, coarse roots (d ≥ 2 mm), and fine roots (d < 2 mm). Error bars show SD among rhizomes (n = 5) and coarse and fine roots (n = 3–4). Different letters indicate significant differences in Si concentrations among each organ and study site for aboveground or underground parts (Tukey’s HSD, p < 0.05)
Figure 5a shows the biomass distribution in each organ at each of the three sites. Among all organs, the culms had the greatest biomasses, and the total masses of underground organs were comparable, accounting for 39–57 % of the total biomass. The site at Noguchi had the most biomass, more than twice that at Kanpachi.
Figure 5b shows the amounts of Si that accumulated in each organ at each site, calculated using the Si concentration and total biomass. We found that more Si accumulated underground than aboveground at Seto and Noguchi, and the values were almost equivalent at Kanpachi. The degree of Si accumulation in culms was almost identical at all three sites. In the litterfall, Si concentration was also highest (51–87 mg/g) in the leaves (Fig. 6). Silicon concentrations in the fresh and litter leaves tended to be lower at Noguchi than at Kanpachi and Seto (Figs. 2, 3a, 6).
Concentration and supply of Si in litter
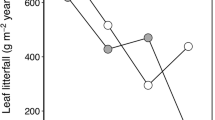
The litterfall that was collected monthly and classified into leaves, leaf sheaths, branches, and other material showed seasonal variation (Fig. 7). Defoliation was greatest from April to June in Kanpachi and Seto. In contrast, litterfall mass was almost constant through the year in Noguchi. The Si concentration in litter leaves was higher than in other organs (Fig. 6), corresponding to the higher distribution of Si in living leaves (Fig. 3). The annual amounts of litterfall are shown in Fig. 8a; the greatest amount was observed at Kanpachi, then Seto, and then Noguchi. From these data, we calculated the annual Si supply through litterfall as shown in Fig. 8b. The Si supplied by leaves was largest, accounting for 72–88 % of the total supply. The difference in annual supply among the sites was distinctly varied; a Si supply of 77 kg/ha/year was observed at Noguchi, much less than the 310 kg/ha/year at Kanpachi or 330 kg/ha/year at Seto.
Discussion
Characteristics of Si concentrations in bamboo organs
We found that the Si concentration in leaves was the highest among all organs and increased with time (Figs. 2, 3), indicating a capacity to accumulate Si. Because plants with Si contents higher than 1.0 % and Si/Ca ratios higher than 1.0 are defined as Si accumulators (Ma and Takahashi 2002), the Si concentration levels (60, 68, 48 mg/g in Kanpachi, Seto, and Noguchi, respectively) and Si/Ca ratio (6.5–10.0, data not shown) clearly indicated that P. pubescens is an Si-accumulating plant. The high concentrations of Si in leaves indicated that this element is transported through the xylem with little deposition in intervening structures, such as culms or branches, and is then accumulated in leaves, which are the endpoint of the transpiration stream. In contrast, in the rhizosphere, Si has been proposed to be absorbed by two mechanisms, active uptake or passively accompanied by transpiration (Liang et al. 2006). Silicon has been shown to be actively taken up as H4SiO4 through the silicic acid transporter (Lsi1, Lsi2) in rice, corn, and barley roots (Ma et al. 2006; Mitani et al. 2009). Although the existence of these transporters has not yet been confirmed in bamboo species, the high concentrations of Si in bamboo leaves suggested active Si uptake using similar transporters.
The possible functions of Si in bamboo plants were previously investigated by Ueda and Ueda (1961), who reported that application of calcium silicate in a Phyllostachys bambusoides forest increased culm hardness. In addition, silicification of the epidermis and vascular tissues enhances the strength and rigidity of the cell wall in gramineous species such as rice (Ma and Takahashi 2002) and in aquatic and wetland species (Schoelynck et al. 2010, 2012). We found a higher concentration of Si in the outer surface of bamboo culms (9.2–14.6 mg/g) than in the inner tissue (0.04–3.8 mg/g) in a preliminary experiment (data not shown), although these values were lower than those in leaves. These facts may indicate that bamboo culms also play a role in structural enhancement by the localized accumulation of Si.
In addition to the physiological mechanisms of Si-accumulating plants, environmental factors are considered to limit Si concentration (Table 3). We hypothesize that a passive mechanism might also be at work, because bamboos have high leaf transpiration rate and high moisture demands (Uchimura 1994; Komatsu et al. 2010). At Noguchi, where the soil water content was lowest (Table 2), leaf Si concentration tended to be lower than that at the other two sites (Figs. 2, 3a, 6). This low soil water content could be attributed to the fact that the high density of bamboo stems (4790 stems/ha) resulted in intense competition for water uptake or to topographical features of the mountain side, in contrast to both Kanpachi and Seto sites, which were located at the base of a mountain and close to water flow. In a previous study, the reduction of transpiration induced a decrease in Si uptake by plants with passive Si uptake (Okuda and Takahashi 1962). In addition, the low soil water content in Noguchi may cause slower dissolution of biogenic silica (e.g., phytolith) or silicate minerals, making root uptake of the dissolved silica more difficult. Although transpiration declines and the solubility of soil biogenic silica due to soil water deficit were not measured in this study, the soil water conditions and bamboo stem density could be important factors controlling Si concentrations in leaves and culms, which differed among sites.
Biogenic Si pools in P. pubescens forests
We found that the underground biomasses in bamboo forests were quite large, especially that of fine roots distributed in the upper soil layer (Figs. 4, 5a). The T/R ratio (the ratio of aboveground to underground biomass) observed in our three study sites ranged from 0.76 to 1.58. These T/R ratios clearly differed from those in conifer or evergreen broadleaved forests (3.0–7.0), but were similar to those in a perennial grass community, which had T/R ratios of less than 1.0 (Saito 1989). The large underground biomass resulted in substantial underground Si accumulation, comparable to or almost exceeding that seen aboveground, even though the highest Si concentration was found in leaves. This result indicated that a huge biotic Si pool existed in the underground tissues of bamboo forests.
Annual Si supply through litterfall was 77–330 kg/ha/year, and 72–88 % of this Si supply was comprised of bamboo leaves (Fig. 8b). Because Si exists as a phytolith (SiO2·nH2O) in the plant body (Kondo 2010), the Si of litter organs is understood to be supplied to the forest soils through decomposition. These phytoliths, mostly comprised of silicic acid (SiO2) (Kondo 2010), could be a source of available Si for bamboo roots and thus circulate within bamboo forests. The amount of phytolith supplied by litterfall in these research sites was calculated at 165 to 706 kg/ha/year by multiplying the Si supply (77–330 kg/ha/year) by 2.14 (wt. SiO2/wt. Si). According to previous studies on various vegetation types, the total amount of phytolith supplied by litterfall is 16–850 kg/ha/year in grasslands, 4–43 kg/ha/year in coniferous forests, and 10–240 kg/ha/year in broadleaved forests (Kondo 1988; Song et al. 2012a, 2013). Comparing our results to these data, a P. pubescens forest clearly circulates a large amount of Si as amorphous silicic acid, and this circulation is more comparable to grasslands than to other forest types.
Estimation of the Si cycle in three bamboo forests
In this study, there were remarkable differences in characteristics among our three research sites. These differences included a high density of bamboo stems, the lowest water content, and lower Si concentrations in leaves and culms at Noguchi. This site was also characterized by having no apparent seasonal change in litterfall (Fig. 7), unlike Kanpachi and Seto, which had peak defoliation from April to June, corresponding to the seasonal changes found by Isagi et al. (1997). This apparent lack of seasonal change and lower amount of litterfall (Fig. 8a) at Noguchi may relate to its high stem density and lowest observed water content or annual variation in litterfall.
In Fig. 9, we summarize the values of Si pools and supply via litterfall for three sites over 1 year. This figure summarizes the Si dynamics in three bamboo forests. The Noguchi forest was characterized by a large biological pool with little Si movement. In contrast, more Si movement with smaller biological pools may be features of the Kanpachi and Seto forests. The Si turnover times calculated from our data were 1.3 and 1.9 years in Kanpachi and Seto, respectively, and 12.2 years in Noguchi. According to research on the Si cycle in natural vegetation dominated by mature P. pubescens stands in China conducted by Li et al. (2006), the turnover time of Si is 16.4 years. From these findings, we suggest that there exists a range of variation in Si turnover rates, which could be explained by annual variation in the P. pubescens litterfall cycle. Li et al. (1998a) observed that the amount of fallen leaves in bamboo forests in China changed annually, however, Suzuki (1976) reported that the majority of leaves in bamboo forests in Japan renewed within 1 year. Although the biocycle of litterfall has not yet been clarified, the study duration at Noguchi might have been a period of lower litterfall, and a greater amount of litterfall from April to June might cause faster Si turnover. Thus, we suggest that there exists a range of variation in Si turnover times (1.3–12.2 years) and Si cycles in P. pubescens forests. This range might depend on forest conditions, such as soil water content or stem density caused by topographical features or management level and also on annual variations in litterfall cycle and stand age, although further research is necessary.
Conclusion
We studied the biological cycle of Si in three P. pubescens stands in central Japan. Total underground organ masses were comparable to culm biomasses and accounted for 39–57 % of the total biomass. Bamboo roots in the surface soil horizon (0–10 cm depth) comprised 54–60 % of the whole root biomass. Ranges of Si accumulation at the three sites were 200–360 kg/ha aboveground and 180–460 kg/ha underground. We found that in moso bamboo stands, a huge biogenic Si pool existed in underground tissues, comparable to that in aboveground tissues. In addition, a large amount of Si, 77–330 kg/ha/year, was supplied through litterfall, 72–88 % of which was comprised of bamboo leaves. The amount of phytolith (SiO2) supplied by litterfall was calculated at 165–706 kg/ha/year. This Si circulation in P. pubescens forests was more comparable to that of grasslands, with more Si, than other forest vegetation types. We observed a range of variation in Si turnover (1.3–12.2 years) from our year-long study in P. pubescens forests.
References
Bakker DNVJ, Hemminga MA, Soelen VJ (1999) The relationship between silicon availability, and growth and silicon concentration of the salt marsh halophyte Spartina anglica. Plant Soil 215:19–27
Bartoli F (1983) The biogeochemical cycle of silicon in 2 temperate forest ecosystems. Ecol Bull 35:469–476
Basile-Doelsch I, Meunier JD, Parron C (2005) Another continental pool in the terrestrial silicon cycle. Nature 433:399–402
Berner RA (1995) Chemical weathering and its effect on atmospheric CO2 and climate. In: White AF, Brantley SL (eds) Chemical weathering rates of silicate minerals, vol 31., Reviews of mineralogyMineralogical Society of America, Washington, DC, pp 565–583
Conley DJ (1997) Riverine contribution of biogenic silica to the oceanic silica budget. Limnol Oceanogr 42:774–777
Conley DJ (2002) Terrestrial ecosystems and the global biogeochemical silica cycle. Global Biogeochem Cycles 16:1–8
Conley DJ, Likens GE, Buso DC, Saccone L, Bailey SW, Johnson CE (2008) Deforestation causes increased dissolved silicate losses in the Hubbard Brook Experimental Forest. Glob Change Biol 14:2548–2554
Cornelis JT, Ranger J, Iserentant A, Delvaux B (2010) Tree species impact the terrestrial cycle of silicon through various uptakes. Biogeochemistry 97:231–245
Derry LA, Kurtz AC, Ziegler K, Chadwick OA (2005) Biological control of terrestrial silica cycling and export fluxes to watersheds. Nature 433:728–731
Dransfield S, Widjaja EA (1995) Plant resources of South-East Asia. No 7: bamboos. Backhuys, Leiden
Drees LR, Wilding LP, Smeck NE, Sankayi AL (1989) Silica in soils: quartz and disordered silica polymorphs. In: Dixon JB, Weed SB (eds) Minerals in soil environments. Soil Science Society of America, Madison, pp 913–974
Embaye K, Weih M, Ledin S, Christersson L (2005) Biomass and nutrient distribution in a highland bamboo forest in southwest Ethiopia: implications for management. For Ecol Manag 204:159–169
Epstein E (1999) Silicon. Annu Rev Plant Biol 50:641–664
Fu MY, Banik RL (1995) Bamboo production systems and their management. In: Proceedings of the Vth international bamboo workshop and the IVth international bamboo congress, Ubud Bali, Indonesia, vol 1, pp 18–33
Fulweiler RW, Nixon SW (2005) Terrestrial vegetation and the seasonal cycle of dissolved silica in a southern New England coastal river. Biogeochemistry 74:115–130
Gérard F, Mayer KU, Hodson MJ, Ranger J (2008) Modelling the biogeochemical cycle of silicon in soils: application to a temperate forest ecosystem. Geochim Cosmochim Acta 72:741–758
Huang Z-T, Li Y-F, Jiang P-K, Chang SX, Song Z-L, Liu J, Zhou G-M (2014) Long-term intensive management increased carbon occluded in phytolith (PhytOC) in bamboo forest soils. Sci Rep 4:3602. doi:10.1038/srep03602
Isagi Y, Kawahara T, Kamo K, Ito H (1997) Net production and carbon cycling in a bamboo Phyllostachys pubescens stand. Plant Ecol 130:41–52
Japan Meteorological Agency (2011) Normal value based on climate statistics at Toyota city. http://www.data.jma.go.jp/obd/stats/etrn/view/nml_amd_ym.php?prec_no=51&block_no=0464&year=&month=&day=&view=. Accessed 28 April 2013 (in Japanese)
Kleinhenz V, Midmore DJ (2001) Aspects of bamboo agronomy. Adv Agron 74:99–153
Komatsu H, Onozawa Y, Kume T, Tsuruta K, Kumagai T, Shinohara Y, Otsuki K (2010) Stand-scale transpiration estimates in a moso bamboo forest: II. Comparison with coniferous forests. For Ecol Manag 260:1295–1302
Kondo R (1988) Soil genesis and ages of soils from the viewpoint of opal phytolith analysis. Pedologist 32:189–203 (in Japanese)
Kondo R (2010) Plant opal pictorial book. A guide to opal phytoliths study by a scanning electron microscope photograph. Hokkaido University Press, Sapporo, p 387 (in Japanese)
Korndörfer GH, Coelho MN, Snyder GH, Mizutani CT (1999) Avaliação de métodos de extração de silicio para solos cultivados com arroz de sequeiro. Rev Bras Ci Solo Viçosa/MG 23:101–106 (in Portuguese)
Kump LR, Brantley SL, Arthur MA (2000) Chemical weathering, atmospheric CO2 and climate. Annu Rev Earth Planet Sci 28:611–667
Li R, Werger MJA, During HJ, Zhong ZC (1998a) Biennial variation in production of new shoots in groves of the giant bamboo Phyllostachys pubescens in Sichuan, China. Plant Ecol 135:103–112
Li R, Werger MJA, During HJ, Zhong ZC (1998b) Carbon and nutrient dynamics in relation to growth rhythm in the giant bamboo Phyllostachys pubescens. Plant Soil 201:113–123
Li ZJ, Lin P, He JY, Yang ZW, Lin YM (2006) Silicon’s organic pool and biological cycle in moso bamboo community of Wuyishan Biosphere Reserve. J Zhejiang Univ Sci B 7:849–857
Liang Y, Hua H, Zhu YG, Zhang J, Cheng C, Römheld V (2006) Importance of plant species and external silicon concentration to active silicon uptake and transport. New Phytol 172:63–72
Lux A, Luxov M, Abe J, Morita S, Inanaga S (2003) Silicification of bamboo (Phyllostachys heterocycla Mitf.) root and leaf. Plant Soil 255:85–91
Ma JF, Takahashi E (2002) Soil, fertilizer, and plant silicon research in Japan. Elsevier Science, Amsterdam, p 281
Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M (2006) A silicon transporter in rice. Nature 440:688–691
Mailly D, Christanty L, Kimmins JP (1997) ‘Without bamboo, the land dies’: nutrient cycling and biogeochemistry of a Javanese bamboo talun-kebun system. For Ecol Manag 91:155–173
Matichenkov VV, Snyder GH (1996) The mobile silicon compounds in some South Florida soils. Eurasian Soil Sci 12:1165–1180
Meunier JD, Colin F, Alarcon C (1999) Biogenic silica storage in soils. Geology 27:835–838
Mitani N, Chiba Y, Yamaji N, Ma JF (2009) Identification and characterization of maize and barley Lsi2-like silicon efflux transporters reveals a distinct silicon uptake system from that in rice. Plant Cell 21:2133–2142
Motomura H, Fujii T (2000) Distribution of silicified cells in the leaf blades of Pleioblastus chino (Franchet et Savatier) Makino (Bambusoideae). Ann Bot 85:751–757
Nishida T (1989) Differences of chemical components in Phyllostachys bambusoides of one to seven years old. Bamboo J 7:1–4 (in Japanese)
Okuda A, Takahashi E (1962) Studies on the physiological role of silicon in crop plant. Part 8 Some examination on the specific behavior of low land rice in silicon uptake. J Sci Soil Manure Jpn 33:217–221 (in Japanese)
Okuda S, Torii A, Ito T, Uemura T, Sasaki T, Ito T, Kimura M, Toyota N, Sado Y, Yamada T, Yamada M, Ito T, Takeuchi I (2007) Take no chijobu genzonryo wo kan-i ni suitei suru. Kenkyu Seika Sensyu, For Forest Prod Res Inst 64:42–43 (in Japanese)
Parr J, Sullivan L, Chen B, Ye G, Zheng W (2010) Carbon bio-sequestration within the phytoliths of economic bamboo species. Glob Change Biol 16:2661–2667
R Development Core Team (2012) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.r-project.org/
Rabosky DL, Sorhannus U (2009) Diversity dynamics of marine planktonic diatoms across the Cenozoic. Nature 457:183–186
Raghubanshi AS (1994) Effect of bamboo harvest on dynamics of nutrient pools, N mineralization, and microbial biomass in soil. Biol Fertil Soils 18:137–142
Raven JA (1983) The transport and function of silicon in plants. Biol Rev 58:179–207
Raven JA, Falkowski PG (1999) Oceanic sinks for atmospheric CO2. Plant Cell Environ 22:741–755
Saito H (1989) Shinrin no genzonryo. In: Tsutsumi T (ed) Shinrin seitaigaku. Asakura Shoten, Tokyo, pp 46–55 (in Japanese)
Schoelynck J, Bal K, Backx H, Okruszko T, Meire P, Struyf E (2010) Silica uptake in aquatic and wetland macrophytes: a strategic choice between silica, lignin and cellulose? New Phytol 186:385–391
Schoelynck J, Bal K, Puijalon S, Meire P, Struyf E (2012) Hydrodynamically mediated macrophyte silica dynamics. Plant Biol 14:997–1005
Schoelynck J, Müller F, Vandevenne F, Bal K, Barão L, Smis A, Opdekamp W, Meire P, Struyf E (2014) Silicon-vegetation interaction in multiple ecosystems: a review. J Veg Sci 25:301–313
Shanmughavel P, Francis K (1997) Balance and turnover of nutrients in a bamboo plantation (Bambusa bambos) of different ages. Biol Fertil Soils 25:69–74
Smis A, Damme S, Struyf E, Clymans W, Wesemael B, Frot E, Vandevenne F, Hoestenberghe T, Govers G, Meire P (2011) A trade-off between dissolved and amorphous silica transport during peak flow events (Scheldt river basin, Belgium): impacts of precipitation intensity on terrestrial Si dynamics in strongly cultivated catchments. Biogeochemistry 106:475–487
Sommer M, Kaczorek D, Kuzyakov Y, Breuer J (2006) Silicon pools and fluxes in soils and landscapes—a review. J Plant Nutr Soil Sci 169:310–329
Song Z, Zhao S, Zhang Y, Hu G, Cao Z, Wong M (2011) Plant impact on CO2 consumption by silicate weathering: the role of bamboo. Bot Rev 77:208–213
Song Z, Liu H, Si Y, Yin Y (2012a) The production of phytoliths in China’s grasslands: implications to the biogeochemical sequestration of atmospheric CO2. Global Change Biol 18:3647–3653
Song Z, Wang H, Strong PJ, Li Z, Jiang P (2012b) Plant impact on the coupled terrestrial biogeochemical cycles of silicon and carbon: implications for biogeochemical carbon sequestration. Earth Sci Rev 115:319–331
Song Z, Liu H, Li B, Yang X (2013) The production of phytolith-occluded carbon in China’s forests: implications to biogeochemical carbon sequestration. Global Change Biol 19:2907–2915
Street-Perrott FA, Barker PA (2008) Biogenic silica: a neglected component of the coupled global continental biogeochemical cycles of carbon and silicon. Earth Surf Proc Land 33:1436–1457
Struyf E, Conley DJ (2012) Emerging understanding of the ecosystem silica filter. Biogeochemistry 107:9–18
Struyf E, Smis A, Damme S, Meire P, Conley DJ (2009) The global biogeochemical silicon cycle. Silicon 1:207–213
Suzuki T (1976) Productivity of Moso-chiku (Phyllostachys edulis) stands. Trans Jpn For Soc 87:223–224 (in Japanese)
Takahashi K (2003) Available silicic acid. In: Standard method of soil analysis. Editorial Board for Standard Methods of Soil Analysis, Hakuyu-sha, Tokyo, pp 161–165 (in Japanese)
Takahashi E (2007) Sakumotsu ni totte keisan towa nani ka. Tokyo, Nobunkyo, p 189 (in Japanese)
Takahashi E, Miyake Y (1976) Distribution of silica accumulator plants in the plant kingdom (1) Monocotyledons. J Sci Soil Manure 47:296–300 (in Japanese)
Tréguer P, Pondaven P (2000) Silica control of carbon dioxide. Nature 406:358–359
Uchimura E (1994) Invitation to bamboos—wonder of biology. Tokyo, Kenseisha, p 188 (in Japanese)
Ueda K, Ueda S (1961) Effect of silicic acid on bamboo growth. Bull Kyoto Univ For 33:79–99 (In Japanese with English summary)
Ueda K, Ueda S, Yakushiji K (1961) On the seasonal changes of the nutrient contents in Madake (Phyllostachys reticulata). Bull Kyoto Univ For 33:55–66 (in Japanese)
Umemura M, Takenaka C (2010) New combination method of wet digestion and gravimetry for Si analysis in bamboo. Chubu For Res 58:165–166 (in Japanese)
Umemura M, Takenaka C (2014) Changes in chemical characteristics of surface soils in Hinoki cypress (Chamaecyparis obtusa) forests induced by the invasion of exotic Moso bamboo (Phyllostachys pubescens) in central Japan. Plant Species Biol. doi:10.1111/1442-1984.12038
Vandevenne FI, Struyf E, Clymans W, Meire P (2012) Agricultural silica harvest: have humans created a new and important loop in the global silica cycle? Front Ecol Environ 10:243–248
Vogt KA, Persson H (1991) Measuring growth and development of roots. In: Lassoie JP, Hinckley TM (eds) Techniques and approaches in forest tree ecophysiology. CRC Press, Boca Raton, pp 477–501
Wollast R, Mackenzie FT (1983) The global cycle of silica. In: Aston SR (ed) Silicon geochemistry and biochemistry. Academic Press, San Diego, pp 39–76
Acknowledgments
We thank Toshio Koike, Yukizumi Takayama, Masaru Koide, Noboru Koike, and Masao Takayama for permission to work on private property in Noguchi. We thank Go Koyama, Takayuki Hukami, and the officers of Toyota, Aichi, for allowing us to use the study site in Kanpachi. In addition, we thank the officers of Aichi Kaisho-no-mori Center for allowing us to use the study site in Seto. We are grateful to Drs. Kazukiyo Yamamoto, Rie Tomioka, and Takafumi Tezuka of Nagoya University for their advice and discussions regarding our research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Umemura, M., Takenaka, C. Biological cycle of silicon in moso bamboo (Phyllostachys pubescens) forests in central Japan. Ecol Res 29, 501–510 (2014). https://doi.org/10.1007/s11284-014-1150-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-014-1150-5