Abstract
Objective
To examine the effects of oral xylitol administration on the femur and mandibular bone of ovariectomized (OVX) rats.
Methods
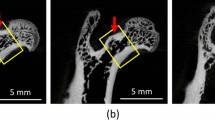
Forty 4-week-old female Wistar rats were randomly divided into two groups of 20 each. One group was subjected to ovariectomy (OVX rats) and the other group to sham surgery (Sham rats). The sham and OVX rats were then subdivided into a group fed only a basal solid diet (n = 10) or a group fed the basal solid diet supplemented with 10% (w/w) xylitol (n = 10). After 40 days on each diet, the rats were tested for serum Ca concentration, alkaline phosphatase (ALP) activity, and tartrate-resistant acid phosphatase (TRAP) concentration as biochemical markers. The morphological analysis involved evaluating the trabecular bone by microfocus X-ray computed tomography. For histological analysis, tissue samples were stained with hematoxylin and eosin.
Results
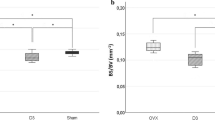
Serum Ca concentration and ALP activity were lower in OVX rats than in Sham rats, and both recovered in rats fed the diet supplemented with xylitol. TRAP concentration was also lower in OVX rats, but decreased still further with xylitol supplementation. OVX rats had a lower bone density in the femur and mandibular bone than Sham rats, and the bone density increased with xylitol supplementation.
Conclusions
Taken together, these findings suggest that dietary oral xylitol administration may influence osteoclasts, with distinct changes in the trabecular bone pattern in the femur and slight changes in the mandibular bone in OVX rats.











Similar content being viewed by others
References
Miyaura C. Estrogen deficiency and postmenopausal osteoporosis. Exp Med. 2002;20:118–23.
Wactawski-Wende J, Hausmann E, Hovey K, Trevisan M, Grossi S, Genco RJ. The association between osteoporosis and alveolar crestal height in postmenopausal women. J Periodontol. 2005;76:2116–24.
Jeffcoat M. The association between osteoporosis and oral bone loss. J Periodontol. 2005;76:2125–32.
Inagaki K, Kurosu Y, Sakano M, Sugiishi T, Yamamoto G, Noguchi T, et al. Osteoporosis and periodontal disease in postmenopausal women: association and mechanisms. Clin Calcium. 2006;16:269–77.
Miller SC, Bowman BM, Miller MA, Bagi CM. Calcium absorption and osseous organ-, tissue-, and envelope-specific changes following ovariectomy in rats. Bone. 1991;12:439–46.
Tanaka M, Ejiri S, Toyooka E, Kohno S, Ozawa H. Effects of ovariectomy on trabecular structures of rat alveolar bone. J Periodont Res. 2001;37:161–5.
Tanaka M, Ejiri S, Nakajima M, Kohno S, Ozawa H. Changes of cancellous bone mass in rat mandibular condyle following ovariectomy. Bone. 1999;25:339–47.
Taguchi A, Tanimoto K, Suei Y, Wada T. Tooth loss and mandibular osteopenia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:127–32.
Yoshihara A, Seida Y, Hanada N, Miyazaki H. A longitudinal study of the relationship between periodontal disease and bone mineral density in community-dwelling older adults. J Clin Periodontol. 2004;31:680–4.
Inagaki K, Kurosu Y, Kamiya T, Kondo F, Yoshinari N, Noguchi T, et al. Low metacarpal bone density, tooth loss and periodontal disease in Japanese women. J Dent Res. 2001;80:1818–22.
Consensus development conference. Diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94:646–50.
Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–8.
Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62:527–34.
Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B, et al. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaw—2009 update. Aust Endod J. 2009;35:119–30.
Lacey JV Jr, Mink PJ, Lubin JH, Sherman ME, Troisi R, Hartge P, et al. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA. 2002;288:334–41.
Barrett-Connor E. Hormone replacement and cancer. Br Med Bull. 1992;48:345–55.
Washuttl J, Reiderer P, Banche E. A qualitative and quantitative study of sugar-alcohols in several foods. J Food Sci. 1973;38:1262–3.
Hollman S, Touster O. Non-glycolytic pathways of metabolism of glucose. New York: Academic Press; 1964.
Mäkinenn KK. Biochemical principles of the use of xylitol in medicine and nutrition with special consideration of dental aspects. Exp Suppl. 1978;(30):1–160.
Mählemann H, Regolati B, Marthaler T. The effect on rat fissure caries of xylitol and sorbitol. Helv Odontol Acta. 1970;14:48–50.
Mäkinenn KK. Enzyme dynamics of a cariogenic streptococcus. The effect of xylitol and sorbitol. J Dent Res. 1972;51:403–8.
Mäkinenn KK, Scheinin A. The effect of the consumption of various sugars on the activity of plaque and salivary enzymes. Int Dent J. 1971;21:331–9.
Mäkinenn KK, Scheinin A. The effect of various sugar and sugar mixtures on the activity and formation of enzymes of dental plaque and oral fluid. Acta Odontol Scand. 1972;30:259–75.
Scheinin A, Mäkinenn KK. The effect of various sugars on the formation and chemical composition of dental plaque. Int Dent J. 1971;21:302–21.
Scheinin A, Mäkinenn KK, Tammisalo E, Rekola M. Turkusugar studies XVIII. Incidence of dental caries in relation to 1-year consumption of xylitol chewing gum. Acta Odontol Scand. 1975;33:269–78.
Loesche WJ, Earnest R, Grossman NS, Corpron R. The effects of chewing gum on the plaque and saliva levels of streptococcus mutans. J Am Dent Assoc. 1984;108:587–91.
Mäkinen KK, Söderling E, Isokangas PJ, Tenovuo J, Tiekso J. Oral biochemical status and depression of Streptococcus mutans in children during 24- to 36-month use of xylitol chewing gum. Caries Res. 1989;23:261–7.
Steinberg LM, Odusola F, Mandel ID. Remineralizing potential, antiplaque and antigingivitis effects of xylitol and sorbitol sweetened chewing gum. Clin Prev Dent. 1992;14:31–4.
Cronin M, Gordon J, Reardon R, Balbo F. Three clinical trials comparing xylitol-and sorbitol-containing chewing gums for their effect on supragingival plaque accumulation. J Clin Dent. 1994;5:106–9.
Mäkinen KK, Isotupa KP, Mäkinen PL, Söderling E, Song KB, Nam SH, et al. Six-month polyol chewing-gum programme in kindergarten-age children. A feasibility study focusing on mutans streptococci and dental plaque. Int Dent J. 2005;55:81–8.
Sato H, Ito H, Murakashi E, Sekino S, Numabe Y. Effects of xylitol-containing chewing gum on plaque formation and salivary components. J Jpn Soc Periodontol. 2008;50:231–7.
Georgieff M, Moldawer LL, Bistrian BR, Blackbum GL. Xylitol, an energy source for intravenous nutrition after trauma. J Parenter Enteral Nutr. 1985;9:199–209.
Uhari M, Kontiokari T, Niemelä M. A novel use of xylitol sugar in preventing acute otitis media. Pediatrics. 1998;102:879–974.
Mattila P, Svanberg M, Knuuttila M. Increased bone volume and bone mineral content in xylitol-fed aged rats. Gerontology. 2001;47:300–5.
Mattila P, Knuuttila M, Kovanen V, Svanberg M. Improved bone biomechanical properties in rats after oral xylitol administration. Calcif Tissue Int. 1999;64:340–4.
Svanberg M, Knuuttila M. The effects of dietary xylitol on recalcifying and newly formed cortical long bone in rats. Calcif Tissue Int. 1993;53:135–8.
Svanberg M, Knuuttila M. Dietary xylitol prevents ovariectomy induced changes of bone inorganic fraction in rats. Bone Miner. 1994;26:81–8.
Mattila P, Svanberg M, Knuuttila M. Diminished bone resorption in rats after oral xylitol administration. A dose-response study. Calcif Tissue Int. 1995;56:232–5.
Svanberg M, Mattila P, Knuuttila M. Dietary xylitol retards the ovariectomy-induced increase of bone turnover in rats. Calcif Tissue Int. 1997;60:462–6.
Sato H, Ide Y, Nasu M, Numabe Y. The effects of oral xylitol administration on bone density in rat femur. Odontology. 2011. 99:28–33.
Otsu N. An automatic threshold selection method based on discriminant and least squares criteria. Trans Inst Electron Commun Eng Jpn. 1980;63:349–56.
Kanri Y, Shimazu Y, Aoba T. Use of microfocus X-ray computer tomography for 3D-image construction and quantitative morphoanalysis. J Oral Biosci. 2004;46:67–73.
Hildebrand T, Ruegsegger P. A new method for the model-independent assessment of thickness in three-dimensional images. J Microsc. 1997;185:67–75.
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, et al. Bone histomorphometry. Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610.
Hahn M, Vogel M, Pompesius-Kempa M, Delling G. Trabecular bone pattern factor. A new parameter for simple quantification of bone microarchitecture. Bone. 1992;13:327–30.
Nishizawa Y. Bone-metabolism marker. Iyaku (Med Drug) J. 2001;16–27.
Hämäläinen MM, Mäkinenn KK, Parviainen MT, Koskinen T. Peroral xylitol increases intestinal calcium absorption in the rat independently of vitamin D action. Miner Electrolyte Metab. 1985;11:178–81.
Knuuttila M, Svanberg M, Hämäläinen MM. Alterations in rat bone composition related to polyol supplementation of the diet. Bone Miner. 1989;6:25–31.
Hämäläinen MM, Knuuttila M, Svanberg M, Koskinen T. Comparison of the effect of glucose, lactose and xylitol on bone recalcification in calcium-deficient rats. Bone. 1990;11:429–38.
Matsumoto T. Molecular biology of bone and osteoporosis. Tokyo: Medical Review Press; 2001.
Minkin C. Bone acid phosphatase. Tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif Tissue Int. 1982;34:285–90.
Oku T, Tanabe K, Watanabe Y, Ono H, Naruse M, Nakamura S. Effects of non-oligosaccharides with different properties on Ca and Mg metabolism in rats. J Jpn Soc Nutr Food Sci. 2007;60:233–40.
Hirama Y, Morohashi T, Sano T, Maki K, Ohta A, Sakai N, et al. Fructo-oligosaccharides prevent disorders of the femoral neck following gastrectomy in growing rats. J Bone Miner Metab. 2003;21:294–8.
Ito M. Analysis of trabecular microstructure using micro-computed tomography. Nippon Rinsho. 1998;56:126–32.
Ferretti JL. Perspectives of pQCT technology associated to biomechanical studies in skeletal research employing rat models. Bone. 1995;17:145–54.
Morita R, Yamamoto I, Takada M, Yuu I, Ohta T, Matsushita R, et al. Recent development and clinical application of bone mineral measurements (in Japanese). Nihon Rinsho. 1998;56:1458–63.
Wakabayashi S, Sakurai T, Kashima I. Relationships between bone strength and bone quality: three-dimensional imaging analysis in ovariectomized mice. Oral Radiol. 2004;20:32–6.
Miyake M, Kozai Y, Sakurai T, Kashima I. Effect of citric acid administration on femoral trabecular structures in ovariectomized mice. Oral Radiol. 2007;23:34–41.
Acknowledgments
We thank Nobuhito Nango (Ratoc System Engineering, Tokyo, Japan) for his technical advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sato, H., Ide, Y., Nasu, M. et al. Effects of xylitol on the femur and mandibular bone in ovariectomized rats. Oral Radiol 28, 38–47 (2012). https://doi.org/10.1007/s11282-011-0079-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11282-011-0079-4