Abstract
Background
Menopause-induced decline in estrogen levels in women is a main factor leading to osteoporosis. The objective of this study was to investigate the effect of intermittent parathyroid hormone (PTH) on bone structural parameters of the femoral neck in ovariectomized rats, in addition to correlations of maximum fracture force.
Methods
Fifteen female Wister rats were divided into three groups: (1) control group; (2) ovariectomized (OVX) group; and (3) OVX + PTH group. All rats were then killed and the femurs extracted for microcomputed tomography scanning to measure volumetric bone mineral density (vBMD) and bone structural parameters of the femoral neck. Furthermore, the fracture forces of femoral neck were measured using a material testing system.
Results
Compared with the control and OVX + PTH groups, the OVX group had significantly lower aBMD, bone parameter, and mechanical strength values. A comparison between OVX and OVX + PTH groups indicated that PTH treatment increased several bone parameters. However, the OVX + PTH groups did not significantly differ with the control group with respect to the bone structural parameters, except for trabecular bone thickness of cancellous bone, which was greater. In addition, among the bone structural parameters, the CSA and BSI of cortical bone were significantly correlated with the maximum fracture force of the femoral neck, with correlations of, respectively, 0.682 (p = 0.005) and 0.700 (p = 0.004).
Conclusion
Intermittent PTH helped treat ovariectomy-induced osteoporosis of cancellous bone and cortical bone in the femoral necks of rats. The ability of the femoral neck to resist fracture was highly correlated with the two parameters, namely cross-sectional area (CSA) and bone strength index (= vBMD × CSA), of cortical bone in the femoral neck and was less correlated with aBMD or other bone structural parameters.
Similar content being viewed by others
Background
Osteoporosis is a common and serious health issue globally [1,2,3,4]. Osteoporosis is serious because continual bone loss results in bone fragility, which, in turn, leads to osteoporotic fracture and subsequent severe pain and difficulty in movement. This decreases the patient’s quality of life. Operation may even be required in some cases, which carries inherent risks of surgery, the discomfort of anesthesia, greater medical expenditure, and a greater burden on the public health-care system [5]. Postmenopausal osteoporosis is common among postmenopausal women; this is because of decreased estrogen levels from decreased ovary function, which results in considerable bone loss, subsequently decreased bone mineral density, and subsequent fragile fractures [6]. According to the literature, approximately 30% of postmenopausal women are affected by osteoporosis, and approximately 40% of this 30% will have experienced one or more fragility fractures in their remaining lifetime [7,8,9].
Specifically, hip fractures are a devastating type of osteoporotic fracture, with approximately 1.6 million patients worldwide every year. The morbidity risk of hip fracture has increased with the rapid aging of the world population. Hip fracture leads to disability, and it has a mortality that is three to four times higher than that in the general population [10]. Furthermore, the mortality of hip fracture 1 year after surgery ranges from 16.6 to 30% [11,12,13]. Half of those patients who survive from a hip fracture lose their functional independence, and a third of them eventually become totally dependent [14], which greatly increases the burden on society [15]. Therefore, osteoporosis-related hip fractures, which are characterized by poor functional outcomes and a high mortality rate, should be prevented through interventions such as exercise and medication.
At present, osteoporosis can be treated by two types of commonly available drugs. The first is antiresorptive drugs, which are first-line drugs whose main function is to suppress osteoclasts. Among antiresorptive drugs, bisphosphonate drugs are the most common. Bisphosphonate drugs can significantly improve bone mineral density in the hips and vertebra body, which, in turn, decreases the probability of fracture [16]. However, the long-term use of bisphosphonate drugs overly suppresses osteoclast function and may be complicated with atypical femoral fractures, osteonecrosis of the jaw (ONJ), gastrointestinal intolerance, acute phase reaction (an influenza-like syndrome) as well as atrial fibrillation [17, 18]. The second type is anabolic drugs, among which parathyroid hormone medications are a specific type. Teriparatide [PTH (1–34), Forteo; Eli Lilly] is a segment of recombinant human PTH peptide. Intermittent teriparatide can effectively treat osteoporosis by (1) increasing the number of osteoblasts, stimulating their activity, and decreasing their apoptosis [19] and (2) increasing bone mass in the lumbar vertebra and long bones, increasing trabecular bone thickness, and bone mineral density in the hips or spine [20].
Bone remodeling, regulated by systemic continuous or intermittent PTH administration, influences the bone strength via altering bone quality including bone turnover, microarchitecture and morphology in addition to bone mineral density (BMD) [21,22,23]. Although many previous studies have elucidated that the provision of intermittent PTH contributes greatly to treating osteoporosis, these animal studies, have focused on changes of BMD or trabecular structures in vertebra body, tibia, or distal femoral metaphysis [24,25,26]; the effects of this anabolic agent on femoral neck were still not very clarified. Furthermore, the hormone-related bone remodeling has site-specific changes that are varied in different skeletal areas [27, 28]. The specific features make it difficult to assume the anabolic effects on other skeletal sites, either trabecular or cortical bone, to femoral neck. Therefore, the proximal femur of the OVX rat might be more clinically relevant to human hip fracture than other skeletal sites (i.e., proximal tibia or distal femur) for preclinical testing of therapeutic agents on OVX-related estrogen-deficiency bone loss. There is still a paucity of evidences on the preventive effects of intermittent PTH used in “early” menopausal stage for the prevention of bone deteriorations, in terms of bone density, microstructural changes and bone strength of femoral neck.
Thus, this study examined how intermittent PTH treatment affects cortical and cancellous bone structural parameters of the femoral neck in ovariectomized rats and how those parameters correlate with mechanical strength. To do so, a material testing system was used to measure the maximum fracture force of the femoral neck and to explore the correlation between such mechanical strength and the bone structural parameters assessed with DXA and micro-CT.
Results
Effect on mechanical strength and bone structure parameters by OVX and intermittent parathyroid hormone treatment
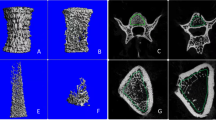
Table 1 lists the data on the aBMD, bone parameters of the femoral necks and mechanical strength of the three groups of rats. Compared with the control and OVX + PTH groups, the OVX group had significantly less mechanical strength of the femoral neck. Although the median of mechanical strength for the control group was 10.3% greater than that for the OVX + PTH group, this difference was not significant. In addition, compared with the control and OVX + PTH groups, the OVX group had a significantly less aBMD of the femoral neck. In essence, the femoral neck of the OVX group was the most cancellous, as revealed in 2D cross-sectional images and particularly for the cortical bone tissue, whereas the difference between the control and OVX + PTH groups was small (Fig. 1).
Coronal-sectional micro-CT images for the a control, b OVX, and c OVX + PTH groups. The images were obtained from one representative sample, and the yellow rectangle depicts the region of interest (ROI) for the measurement of the femoral neck. Thinning of cortical bone (red arrow) after OVX and regaining of thickness in the PTH treatment group
The following are the results for the four cancellous bone parameters. BV/TV did not significantly differ between the control and OVX groups and between the control and OVX + PTH groups; however, BV/TV was significantly greater in the OVX + PTH group than in the OVX group. TbSp and TbN did not significantly differ between the three groups. TbTh was greater in the OVX + PTH group than in the control and OVX groups.
The following are the results for the four cortical bone parameters. CtTh and vBMD did not significantly differ between the three groups. CSA did not significantly differ between the control and OVX groups or between the control and OVX + PTH groups; however, CSA was significantly greater in the OVX + PTH group than in the OVX group. BSI did not significantly differ between the control and OVX + PTH groups; BSI in both these groups was greater than that in the OVX group.
Correlations of aBMD and cortical and bone parameters with maximum fracture force of the femoral neck
According to Table 2, the aBMD and trabecular bone structural parameters were not significantly correlated with the maximum fracture force of the femoral neck (p > 0.05). Moreover, among the cortical bone parameters, CtTh and vBMD were not correlated with the maximum fracture force of the femoral neck (p > 0.05). However, both CSA (0.682, p = 0.005) and BSI (0.700, p = 0.004) were highly correlated with the maximum fracture force of the femoral neck. In addition, no significant correlation was found between the aBMD and vBMD (r = − 0.213, p = 0.445).
Discussion
Osteoporosis has become more prevalent as societies have aged. Patients with osteoporosis face a higher risk of fractures and a decreased quality of life. Studies have suggested that in addition to antiresorptive drugs with bisphosphonates, anabolic drugs, such as those in intermittent PTH treatment, effectively treat osteoporosis. However, studies have mostly investigated the vertebra body or long bone [29,30,31,32]. Findings on how intermittent PTH treatment improves (1) the femoral neck bone structure or (2) the resistance of the femoral neck against fractures remain inadequate. This study used micro-CT to explore ovariectomy-induced osteoporosis in rats. Specifically, this study focused on how intermittent PTH treatment changed the cortical bone and cancellous bone structures of the femoral neck and how cortical bone and cancellous bone parameters were correlated with the ability of the femoral neck to resist fracture. The results indicated that intermittent PTH contributed to treating ovariectomized-induced osteoporosis in the cancellous bone and in the cortical bone of the femoral neck of rats. Femoral neck fracture strength was highly correlated with two parameters of the femoral neck (CSA and BSI) and no correlated with other BMD or bone structural parameters.
Previous studies on osteoporosis have mostly used rats or rabbits for animal experiments [33, 34], and the use of female rats has been particularly common in studies on ovariectomy to simulate menopause-induced osteoporosis in women [35]. A study suggested that rats reach sexual maturity at approximately 6 weeks old [33]. Thus, this study adopted female rats, which were bilaterally ovariectomized at the age of 8 weeks. Teriparatide, hPTH (1–34), is an effective anabolic agent for treating osteoporosis and daily use for 18 months has been recommended to treat osteoporosis in menopausal women. However, the optimal duration of its therapeutic effect on the bones of the ovariectomized rats has no consensus. The period of the intermittent PTH was varied from 6 to 16 weeks to evaluate the effects on bones in the previous studies [36,37,38,39,40]. The 12 weeks of teriparatide use for evaluating the bone changes in the OVX animal model was accepted and commonly used [36, 37, 40]. In this study, we adopted 12-week teriparatide injection to prevent the bone loss from the ongoing estrogen deficiency-related bone deteriorations after OVX. The rats of the treated group were treated with intermittent PTH for 12 weeks, and the corresponding changes in bone mineral density in the femoral neck, in bone structural parameters, and in fracture strength were measured.
This study first adopted DXA, the most commonly used method in clinical practice, to measure bone mineral density. However, DXA can only measure aBMD in two dimensions, whereas bone structure is in three dimensions; two dimensional aBMD measurements cannot completely represent bone strength. This study also used micro-CT to measure the cortical- and cancellous-bone structural parameters of the femoral neck. In general, micro-CT is the gold standard for estimating bone morphology and the microstructure. Micro-CT can be used to measure many parameters of the trabecular bone microarchitecture. This study adopted BV/TV, TbTh, TbSp, and TbN because they are, according to Bouxsein et al. the four representative indicators of trabecular bone microarchitecture [41]. Similarly, the four parameters of cortical bone were adopted because they have been suggested by previous studies to be representative. Specifically, CtTh and CSA are morphological parameters of the femoral neck; vBMD is a density parameter of the cortical bone of the femoral neck; and BSI reflects the state of health jointly indicated by the morphological parameters and density parameters. Regarding the measurements of bone mineral density, no significant correlation between the aBMD and vBMD was found. The possible reasons leading to this diversity might be the inconsistent measured dimensions, 2D in aBMD or 3D in vBMD, and varied ROI. The grabbed ROI of aBMD from DXA was the proximal femur, including femoral the head and neck, whereas the ROI of vBMD focused on the cortical bone at the femoral neck as in the area shown in Fig. 1.
This study’s results agree with those of studies on the trabecular bone microarchitecture of the femur in ovariectomized rats and normal rats [30, 31]. In the present study, the trabecular bone microarchitecture in the OVX group was more cancellous than that in the control group. However, this study differs from the literature with respect to findings on the parameters of trabecular bone microarchitecture in the femur for the OVX and control groups. This difference was probably due to the differences in the breed and the age of rats as well as in the length of the post-ovariectomy period. More importantly, micro-CT measures different bone regions. Studies have mostly examined the trabecular bone microarchitecture in the distal femoral metaphysis [30, 31], whereas this study examined the cancellous bone structure of the femoral neck. Despite the aforementioned differences, however, this study’s observation that the bone structure of the OVX group was significantly more cancellous than that of the control group are consistent with those of previous studies.
Clinical and animal experiments have suggested that intermittent PTH can (1) treat ovariectomy-induced osteoporosis, (2) improve the bone mineral density of the lumbar vertebra and the femur, (3) improve the microarchitecture of the trabecular bone and cortical bone, and (4) decrease the risk of fractures [42,43,44]. Using micro-CT, Washimi et al. [31] observed changes in the trabecular bone microarchitecture of the distal femoral metaphysis in ovariectomized rats treated with intermittent PTH; specifically, in their OVX group, intermittent PTH increased BV/TV (by 48.7%), TbN (by 20.5%), and TbTh (by 22.7%) and decreased TbSp (by 24.3%). Such experimental results are similar to those in the present study on the OVX group trabecular bone parameters of the femoral neck. However, in the OVX + PTH group of the present study, BV/TV and TbTh were significantly higher (by 24.3% and 21.1%, respectively) compared with the values for the OVX group; TbSp and TbN did not significantly differ. This was probably due to the diverse trabecular bone distribution ratios of different body parts [45] or to the different reactions of various body parts to intermittent PTH treatment; these explanations require verification in future studies. Nonetheless, due to estrogen deficiency, intermittent PTH treatment can improve the trabecular bone loss due to estrogen deficiency of the femoral neck (as indicated by the results for BV/TV and TbTh).
In addition, intermittent PTH treatment can accelerate cortical bone formation in the tibial midshaft as well as increase the area and strength of the cortical bone [46]. A human cadaveric study by Werner et al. suggested that the bone mineral content in the femoral neck only accounted for 23.5% [47] and that the contribution of the cancellous bone to the mechanical strength of the femoral neck was marginal; these findings are attributable to most of the load being borne by the cortical bone [48, 49]. Furthermore, in a previous study, when the cancellous bone in the femoral neck was hollowed, the load to fracture of the femoral neck only decreased by 7% [50]. These studies have revealed that changes in the cortical bone in the femoral neck play an important role in mechanical strength. However, other studies have furnished opposing findings that the cancellous bone is related to femoral neck strength [51, 52]. Evidence on the correlation between cortical bone parameters and femoral neck strength remain somewhat conflicting, and how medical treatment changes the cortical bone and mechanical strength of the femoral neck remain to be clarified. Moreover, due to the particular shape of the femoral neck, its strength is affected not only by bone mineral density, but also by geometric shape and cortical bone microarchitecture, including parameters such as bone surface CSA, cross-sectional moment of inertia, and cortical thickness [53]. The results of this study indicated that although aBMD and the parameters of trabecular bone are not significantly correlated with mechanical strength, the mechanical strength is highly correlated with the CSA and BSI of the cortical bone. Consistent with those of previous studies, this study’s findings indicate the importance of the cortical bone to the femoral neck with respect to mechanical strength. From the statistical analysis, the BSI, which is multiplication of vBMD and CSA, was highly correlated with maximum fracture force of the femoral neck. This positive correlation between BSI and mechanical strength of the femoral neck might be caused by the direct relation of maximum fracture load to CSA, but not vBMD. Furthermore, we also found that the enhanced mechanical strength in the femoral necks of the rats of the OVX + PTH group was probably due to the changes produced through intermittent PTH treatment in the cortical bone structure of the femoral neck. Several studies have demonstrated the curative effect of intermittent PTH treatment on osteoporosis of the femoral neck. However, few studies have examined the correlations of intermittent PTH with microstructural changes in the femoral neck and with mechanical strength, and further research on this topic is required.
This study has the following limitations. First, this animal study did not involve human participants. Second, the sample size for each group was five, slightly lower than that in previous studies, in which six bone specimens were included in each group [54,55,56]. Third, this study did not examine other bone biochemical indices through a blood test; it only used micro-CT scans to estimate bone structural parameters. Fourth, this study did not measure the parameters of other bones with frequent osteoporosis-induced fractures, such as the vertebra body or radius; it only measured the bone structural parameters of the femoral neck.
Conclusions
Intermittent PTH helped treat ovariectomy-induced osteoporosis of the cancellous bone and cortical bone in the femoral necks of rats. The ability of the femoral neck to resist fracture was highly correlated with two parameters, namely the CSA and BSI of the cortical bone in the femoral neck, and it was less correlated with aBMD or other bone structural parameters.
Materials and methods
Animal preparation and experimental design
For this study’s experiment, animal research ethics approval was obtained from the Research Ethics Committee of the Taichung Veterans General Hospital (Permit Number: La1031191). All experiments were conducted in accordance with relevant guidelines and regulations. This study adopted fifteen 8-week-old female Wistar rats, which were divided into three groups of five rats each. These groups were the (1) control group, where the rats were not ovariectomized and were raised to the age of 20 weeks; (2) ovariectomized group (OVX group), where the rats were bilaterally ovariectomized at the age of 8 weeks and raised for the following 12 weeks to simulate postmenopausal osteoporosis in women; and (3) treated group (OVX + PTH group), where the rats were bilaterally ovariectomized at the age of 8 weeks, followed by an intermittent treatment with Forteo®, a recombinant human PTH with the 34 amino-terminal amino acids, for the following 12 weeks (injection of 50 μg/kg twice weekly). Intermittent PTH injections were performed subcutaneously in those OVX animals of treated group commencing the day after surgery. After the period of 3-month intervention, all the rats were killed at the age of 20 weeks, and their right femur was extracted for further testing. All the removed bones were covered with gauze soaked in 0.9% saline before immediately being preserved in a refrigerator at − 20 °C. We devoted to avoid the bones’ dehydration during the whole experiment. All specimens were kept well after thawing and each of them was separately covered in saline-soaked gauze as well as packed well in zipper storage bags to prevent the dehydration during the whole experiment. These bone specimens were only defrosted to room temperature before the examinations were performed. The extracted bones were subjected to the DXA and micro-CT test first in 1 week and then they were set up to complete biomechanical test in another week. All of the tests were completed in 2 weeks. The collected data were further analyzed.
Measurement of mineral density of areal bone
Frozen samples were defrosted to room temperature prior to DXA measurement. A single qualified DXA technician conducted all examinations for all samples. All extracted femurs were measured using a fan beam densitometer designed for clinical use (Lunar Prodigy Advanced System, General Electric, Madison, WI, USA), which was calibrated daily according to the manufacturer’s instructions. A DXA machine has two X-ray beams with different energy levels, which are aimed at the target bones. The bone mineral density (BMD) can be calculated based on the absorption of each beam by the bone, while soft tissue absorption is subtracted out. Because the whole femurs of experimental rats were extracted without the enveloping soft tissue, a PMMA plate had to be placed underneath the femoral bone to simulate the density of surrounding soft tissue in a DXA scan, according to the manufacturer’s instructions. All specimens were manually placed on an 8-mm-thick polymethylmethacrylate (PMMA) plate to simulate surrounding soft tissue before being scanned individually per the manufacturer’s recommendation. The scan was set to be in the small animal mode, and a laser beam helped mark the start of each scan. The whole femur was scanned using Prodigy DXA. The aBMD of the region of interest (ROI) of the proximal femur, including the femoral head and the neck proximal to the lowest margin of the lesser trochanter, was analyzed using enCORE software (2007, version 11.20.068).
Microcomputed tomography scanning and measurements of bone parameters
Frozen samples were defrosted to room temperature prior to the micro-computed tomography (micro-CT) scan. The micro-CT images of each femur were obtained using a Skyscan 1076 micro-CT device (Aartselaar, Belgium; Fig. 2). The scanning parameters were set as follows: tube voltage, 49 kV; current, 200 μA; exposure time, 500 ms; and voxel resolution, 18.27 μm. The micro-CT scanning images were saved in the TIF file format. After scanning was conducted, the images were imported into NRecon (SkyScan, Aartselaar, Belgium) for image reconstruction. The micro-CT images were imported into CTAn software (Skyscan) to measure the cortical bone and trabecular bone parameters of the femoral neck. The global threshold value for obtaining binary images of the mineralized trabecular bone and the ROIs of the cortical bone and cancellous bone were segmented manually. The following four parameters of trabecular bone microstructure in the femoral neck were measured: bone volume fraction (bone volume/total volume, BV/TV, unit: %), trabecular bone thickness (TbTh, unit: mm), trabecular bone separation (TbSp, unit: mm), and trabecular bone number (TbN, unit: mm−1) [41, 57]. In addition, the four parameters of the cortical bone in the femoral neck were also measured: cortical bone thickness (CtTh), volumetric bone mineral density (vBMD), cross-sectional area (CSA), and bone strength index (BSI, where BSI = vBMD × CSA) [41].
Femoral neck fracture test
Pliers were used to cut off the middle section of the femur to obtain the proximal femur. Generally, the proximal femur was retained about 2 cm in length and temporarily fixed vertically in the container. Then the epoxy resin was used to embed and fix approximately 1 cm away from the distal end of the proximal femur [58,59,60], which was then placed in a material testing system (JSV-H1000, Japan Instrumentation System, Nara, Japan; Fig. 3a). The displacement control mode was used to impose a downward force at a speed of 5 mm/min on the femoral head until the femoral neck fractured (Fig. 3b). The fracture pattern on the femoral neck of all specimens was similar, which was oblique transcervical femoral neck fracture (Fig. 3c). During the experiment, the maximum fracture force was recorded.
Statistical analysis
The data for aBMD, maximum fracture force of the femoral neck, and cancellous and cortical bone parameters of the femoral neck in the three groups were summarized in terms of the median and interquartile range (IQR). A Kruskal–Wallis test was used to analyze differences in CtTh, BV/TV, TbTh, TbSp, and TbN in the femoral neck between the three groups; Mann–Whitney exact tests were used for post hoc pairwise comparisons; and Spearmen’s correlation coefficients (r values) of maximum fracture force with the parameters for the femoral neck and bone structure (aBMD, CtTh, vBMD, CSA, BSA, BV/TV, TbTh, TbSp, and TbN) were calculated. All statistical analyses were performed using SPSS Version 19 (IBM Corporation, Armonk, NY, USA). The level of the statistical significance was set at p < 0.05.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- pths:
-
Parathyroid hormones
- OVX:
-
Ovariectomized
- DXA:
-
Dual-energy X-ray absorptiometry
- abmd:
-
Areal bone mineral density
- ctth:
-
Cortical bone thickness
- vbmd:
-
Volumetric bone mineral density
- CSA:
-
Cross-sectional area
- BSI:
-
Bone strength index
- BV/TV:
-
Bone volume fraction
- tbth:
-
Trabecular thickness
- tbsp:
-
Trabecular separation
- tbn:
-
Trabecular number
- PMMA:
-
Polymethylmethacrylate
- micro-CT:
-
Micro-computed tomography
References
Melton LJ. Epidemiology worldwide. Endocrinol Metab Clin. 2003;32(1):1–13.
Vijayakumar R, Büsselberg D. Osteoporosis: an under-recognized public health problem: local and global risk factors and its regional and worldwide prevalence. J Local Glob Health Sci. 2016;2016(1):2.
Özkal FM, Cakir F, Sensoz E. Schematization of cannulated screw fixations in femoral neck fractures using genetic algorithm and finite element method. J Med Biol Eng. 2020;40:673–87.
Luo Y, Wu X. Bone quality is dependent on the quantity and quality of organic–inorganic phases. J Med Biol Eng. 2020. https://doi.org/10.1007/s40846-020-00506-x.
Borgström F, Karlsson L, Ortsäter G, Norton N, Halbout P, Cooper C, Lorentzon M, McCloskey EV, Harvey NC, Javaid MK. Fragility fractures in Europe: burden, management and opportunities. Arch Osteoporos. 2020;15:1–21.
van Oostwaard M. Osteoporosis and the nature of fragility fracture: an overview. In: Fragility fracture nursing. Cham: Springer; 2018. p. 1–13.
Melton LJ 3rd, Chrischilles EA, Cooper C, Lane AW, Riggs BL. Perspective. How many women have osteoporosis? J Bone Miner Res. 1992;7(9):1005–10.
Reginster JY, Burlet N. Osteoporosis: a still increasing prevalence. Bone. 2006;38(2 Suppl 1):S4-9.
Rungruangbaiyok C, Azari F, van Lenthe GH, Vander Sloten J, Tangtrakulwanich B, Chatpun S. Finite element investigation of fracture risk under postero-anterior mobilization on a lumbar bone in elderly with and without osteoporosis. J Med Biol Eng. 2021. https://doi.org/10.1007/s40846-021-00607-1.
Dubljanin-Raspopović E, Marković-Denić L, Marinković J, Nedeljković U, Bumbaširević M. Does early functional outcome predict 1-year mortality in elderly patients with hip fracture? Clin Orthopaed Relat Res. 2013;471(8):2703–10.
Lee T-C, Ho P-S, Lin H-T, Ho M-L, Huang H-T, Chang J-K. One-year readmission risk and mortality after hip fracture surgery: a national population-based study in Taiwan. Aging Dis. 2017;8(4):402.
Mariconda M, Costa G, Cerbasi S, Recano P, Aitanti E, Gambacorta M, Misasi M. The determinants of mortality and morbidity during the year following fracture of the hip: a prospective study. Bone Jt J. 2015;97(3):383–90.
Morri M, Ambrosi E, Chiari P, Magli AO, Gazineo D, D’Alessandro F, Forni C. One-year mortality after hip fracture surgery and prognostic factors: a prospective cohort study. Sci Rep. 2019;9(1):1–7.
Kannus P, Niemi S, Parkkari J, Palvanen M, Vuori I, Järvinen M. Hip fractures in Finland between 1970 and 1997 and predictions for the future. Lancet. 1999;353(9155):802–5.
Leal J, Gray A, Prieto-Alhambra D, Arden NK, Cooper C, Javaid MK, Judge A, Group RS. Impact of hip fracture on hospital care costs: a population-based study. Osteoporos Int. 2016;27(2):549–58.
Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009;122(2):S14–21.
Nuti R, Brandi ML, Checchia G, Di Munno O, Dominguez L, Falaschi P, Fiore CE, Iolascon G, Maggi S, Michieli R. Guidelines for the management of osteoporosis and fragility fractures. Intern Emerg Med. 2019;14(1):85–102.
Wang J-C, Chien W-C, Chung C-H, Liao W-I, Tsai S-H. Adverse cardiovascular effects of nitrogen-containing bisphosphonates in patients with osteoporosis: a nationwide population-based retrospective study. Int J Cardiol. 2016;215:232–7.
Nakajima M, Ejiri S, Tanaka M, Toyooka E-I, Kohno S, Ozawa H. Effect of intermittent administration of human parathyroid hormone (1–34) on the mandibular condyle of ovariectomized rats. J Bone Miner Metab. 2000;18(1):9–17.
Osagie-Clouard L, Sanghani A, Coathup M, Briggs T, Bostrom M, Blunn G. Parathyroid hormone 1–34 and skeletal anabolic action: the use of parathyroid hormone in bone formation. Bone Jt Res. 2017;6(1):14–21.
Compston J. Bone quality: what is it and how is it measured? Arq Bras Endocrinol Metabol. 2006;50:579–85.
Hadjidakis DJ, Androulakis II. Bone remodeling. Ann NY Acad Sci. 2006;1092(1):385–96.
Fonseca H, Moreira-Gonçalves D, Coriolano H-JA, Duarte JA. Bone quality: the determinants of bone strength and fragility. Sports Med. 2014;44(1):37–53.
Curtis RC, Custis JT, Ehrhart NP, Ehrhart E, Condon KW, Gookin SE, Donahue SW. Combination therapy with zoledronic acid and parathyroid hormone improves bone architecture and strength following a clinically-relevant dose of stereotactic radiation therapy for the local treatment of canine osteosarcoma in athymic rats. PloS ONE. 2016;11(6):e0158005.
Roberts BC, Carrera HMA, Zanjani-pour S, Boudiffa M, Wang N, Gartland A, Dall’Ara E. PTH (1–34) treatment and/or mechanical loading have different osteogenic effects on the trabecular and cortical bone in the ovariectomized C57BL/6 mouse. Sci Rep. 2020;10(1):1–16.
Yoshioka Y, Yamachika E, Nakanishi M, Ninomiya T, Akashi S, Kondo S, Moritani N, Kobayashi Y, Fujii T, Iida S. Intermittent parathyroid hormone 1–34 induces oxidation and deterioration of mineral and collagen quality in newly formed mandibular bone. Sci Rep. 2019;9(1):1–10.
López-Aguirre C, Wilson LA, Koyabu D, Tu VT, Hand SJ. Variation in cross-sectional shape and biomechanical properties of the bat humerus under Wolff’s law. Anat Rec. 2021. https://doi.org/10.1002/ar.24620.
Yamaura M, Nakamura T, Tsurukami H, Hijioka A, Narusawa K, Ohnishi H, Ohta T, Hosoda K. Local bone turnover in the metaphysis of the proximal tibia and the lumbar vertebra during the early periods after ovariectomy in rats. Calcif Tissue Int. 1996;58(1):52–9.
Brouwers J, van Rietbergen B, Huiskes R, Ito K. Effects of PTH treatment on tibial bone of ovariectomized rats assessed by in vivo micro-CT. Osteoporos Int. 2009;20(11):1823–35.
Leitner MM, Tami AE, Montavon PM, Ito K. Longitudinal as well as age-matched assessments of bone changes in the mature ovariectomized rat model. Lab Anim. 2009;43(3):266–71.
Washimi Y, Ito M, Morishima Y, Taguma K, Ojima Y, Uzawa T, Hori M. Effect of combined humanPTH(1–34) and calcitonin treatment in ovariectomized rats. Bone. 2007;41(5):786–93.
Kitaguchi K, Kashii M, Ebina K, Kaito T, Okada R, Makino T, Etani Y, Ishimoto T, Nakano T, Yoshikawa H. The combined effects of teriparatide and anti-RANKL monoclonal antibody on bone defect regeneration in ovariectomized mice. Bone. 2020;130:115077.
Sengupta P. The laboratory rat: relating its age with human’s. Int J Prev Med. 2013;4(6):624–30.
Permuy M, López-Peña M, Muñoz F, González-Cantalapiedra A. Rabbit as model for osteoporosis research. J Bone Miner Metab. 2019. https://doi.org/10.1007/s00774-019-01007-x.
Yousefzadeh N, Kashfi K, Jeddi S, Ghasemi A. Ovariectomized rat model of osteoporosis: a practical guide. EXCLI J. 2020;19:89.
Ma YL, Zeng QQ, Porras LL, Harvey A, Moore TL, Shelbourn TL, Dalsky GP, Wronski TJ, Aguirre JI, Bryant HU. Teriparatide [rhPTH (1–34)], but not strontium ranelate, demonstrated bone anabolic efficacy in mature, osteopenic, ovariectomized rats. Endocrinology. 2011;152(5):1767–78.
Iwamoto J, Seki A, Sato Y. Effect of combined teriparatide and monthly minodronic acid therapy on cancellous bone mass in ovariectomized rats: a bone histomorphometry study. Bone. 2014;64:88–94.
Komrakova M, Hoffmann D, Nuehnen V, Stueber H, Wassmann M, Wicke M, Tezval M, Stuermer K, Sehmisch S. The effect of vibration treatments combined with teriparatide or strontium ranelate on bone healing and muscle in ovariectomized rats. Calcif Tissue Int. 2016;99(4):408–22.
Sugie-Oya A, Takakura A, Takao-Kawabata R, Sano H, Shimazu Y, Isogai Y, Yamaguchi A, Ishizuya T. Comparison of treatment effects of teriparatide and the bisphosphonate risedronate in an aged, osteopenic, ovariectomized rat model under various clinical conditions. J Bone Miner Metab. 2016;34(3):303–14.
Weigl M, Kocijan R, Ferguson J, Leinfellner G, Heimel P, Feichtinger X, Pietschmann P, Grillari J, Zwerina J, Redl H. Longitudinal changes of circulating miRNAs during bisphosphonate and teriparatide treatment in an animal model of postmenopausal osteoporosis. J Bone MinerRes. 2021. https://doi.org/10.1016/j.bone.2019.115104.
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25(7):1468–86.
Hodsman AB, Bauer DC, Dempster DW, Dian L, Hanley DA, Harris ST, Kendler DL, McClung MR, Miller PD, Olszynski WP, et al. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005;26(5):688–703.
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–41.
Tashjian AH, Gagel RF. Teriparatide [human PTH(1–34)]: 25 years of experience on the use and safety of the drug for the treatment of osteoporosis. J Bone Miner Res. 2006;21(3):354–65.
Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(Supplement 3):S131–9.
Mashiba T, Burr DB, Turner CH, Sato M, Cain RL, Hock JM. Effects of human parathyroid hormone (1–34), LY333334, on bone mass, remodeling, and mechanical properties of cortical bone during the first remodeling cycle in rabbits. Bone. 2001;28(5):538–47.
Werner C, Iversen B, Therkildsen M. Contribution of the trabecular component to mechanical strength and bone mineral content of the femoral neck. An experimental study on cadaver bones. Scand J Clin Lab Investig. 1988;48(5):457–60.
Koivumäki JE, Thevenot J, Pulkkinen P, Kuhn V, Link TM, Eckstein F, Jämsä T. Cortical bone finite element models in the estimation of experimentally measured failure loads in the proximal femur. Bone. 2012;51(4):737–40.
Nawathe S, Nguyen BP, Barzanian N, Akhlaghpour H, Bouxsein ML, Keaveny TM. Cortical and trabecular load sharing in the human femoral neck. J Biomech. 2015;48(5):816–22.
Holzer G, Von Skrbensky G, Holzer LA, Pichl W. Hip fractures and the contribution of cortical versus trabecular bone to femoral neck strength. J Bone Miner Res. 2009;24(3):468–74.
Bouxsein ML, Fajardo RJ. Cortical stability of the femoral neck and hip fracture risk. Lancet. 2005;366(9496):1523–4.
Manske S, Liu-Ambrose T, Cooper D, Kontulainen S, Guy P, Forster B, McKay H. Cortical and trabecular bone in the femoral neck both contribute to proximal femur failure load prediction. Osteoporos Int. 2009;20(3):445–53.
Bonnick SL. Hsa: beyond bmd with dxa. Bone. 2007;41(1):S9–12.
Tanaka M, Toyooka E, Kohno S, Ozawa H, Ejiri S. Long-term changes in trabecular structure of aged rat alveolar bone after ovariectomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95(4):495–502.
Irie K, Sakakura Y, Tsuruga E, Hosokawa Y, Yajima T. Three-dimensional changes of the mandible and alveolar bone in the ovariectomized rat examined by micro-focus computed tomography. J Jpn Soc Periodontol. 2004;46(4):288–93.
Bagi CM, Hanson N, Andresen C, Pero R, Lariviere R, Turner CH, Laib A. The use of micro-CT to evaluate cortical bone geometry and strength in nude rats: correlation with mechanical testing, pQCT and DXA. Bone. 2006;38(1):136–44.
Hsu JT, Wang SP, Huang HL, Chen YJ, Wu J, Tsai MT. The assessment of trabecular bone parameters and cortical bone strength: a comparison of micro-CT and dental cone-beam CT. J Biomech. 2013;46(15):2611–8.
Chon C-S, Yun H-S, Kim HS, Ko C. Elastic modulus of osteoporotic mouse femur based on femoral head compression test. Appl Bionics Biomech. 2017. https://doi.org/10.1155/2017/7201769.
Monzem S, Ballester RY, Javaheri B, Poulet B, Sônego DA, Pitsillides AA, Souza RL. Long-term bisphosphonate treatment coupled with ovariectomy in mice provokes deleterious effects on femoral neck fracture pattern and modifies tibial shape. Bone Jt Open. 2020;1(9):512–9.
Brzóska MM, Roszczenko A, Rogalska J, Gałażyn-Sidorczuk M, Mężyńska M. Protective effect of chokeberry (Aronia melanocarpa L.) extract against cadmium impact on the biomechanical properties of the femur: a study in a rat model of low and moderate lifetime women exposure to this heavy metal. Nutrients. 2017;9(6):543.
Acknowledgements
The authors greatly appreciate the invaluable intellectual feedback of Ms. Pei-Yu Hsu from the Master’s Program in Biomedical Engineering, China Medical University and Prof. Fu-Chou Cheng from the Stem Cell Medical Research Center, Department of Medical Research, Taichung Veterans General Hospital.
Funding
This research was partially supported by China Medical University, Taiwan (Grant number: CMU109-MF-117) and Taichung Veterans General Hospital (Grant number: TCVGH-1105104B).
Author information
Authors and Affiliations
Contributions
Conceptualization, W-PW and J-TH; methodology, W-PW, Y-JC, C-EH, Y-CC, M-TT and J-TH; writing—review and editing, W-PW and J-TH; funding acquisition, J-TH. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
For this study’s experiment, animal research ethics approval was obtained from the Research Ethics Committee of the Taichung Veterans General Hospital (Permit Number: La1031191).
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, SP., Chen, YJ., Hsu, CE. et al. Intermittent parathyroid hormone treatment affects the bone structural parameters and mechanical strength of the femoral neck after ovariectomy-induced osteoporosis in rats. BioMed Eng OnLine 21, 6 (2022). https://doi.org/10.1186/s12938-022-00978-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12938-022-00978-9