Abstract
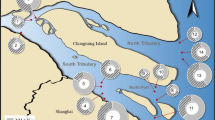
Diversity and distribution pattern of ammonia-oxidizing archaea (AOA) were studied across a salinity gradient in the water column of Cochin Estuary (CE), a tropical monsoonal estuary along the southeast Arabian Sea. The water column of CE was found to be nutrient rich with high bacterial (3.7–6.7 × 108 cells L−1) and archaeal abundance (1.9–4.5 × 108 cells L−1). Diversity and seasonal variation in the distribution pattern of AOA were studied using clone library analysis and Denaturing gradient gel electrophoresis (DGGE). Clone library analysis of both the amoA and 16S rRNA gene sequences showed similar diversity pattern, however the diversity was more clear when the 16S rRNA gene sequences were analyzed. More than 70% of the sequences retrieved were clustered under uncultured Thaumarchaeota group 1 lineage and the major fractions of the remaining sequences were grouped into the Nitrosopumilus lineage and Nitrosopelagicus lineage. The AOA community in the CE was less adaptable to changing environmental conditions and its distribution showed seasonal variations within the DGGE banding pattern with higher diversity during the pre-monsoon period. The distribution of AOA also showed its preference to intermediate salinity for their higher diversity. Summer monsoon associated runoff and flushing played a critical role in regulating the seasonality of AOA distribution.





Similar content being viewed by others
References
Amon RM, Benner R (1996) Bacterial utilization of different size classes of dissolved organic matter. Limnol Oceanogr 41:41–51
Balachandran KK, Reddy GS, Revichandran C, Srinivas K, Vijayan PR, Thottam TJ (2008) Modelling of tidal hydrodynamics for a tropical ecosystem with implications for pollutant dispersion (Cohin Estuary, Southwest India). Ocean Dyn 58:259–273
Bano N, Ruffin S, Ransom B, Hollibaugh JT (2004) Phylogenetic composition of Arctic Ocean archaeal assemblages and comparison with Antarctic assemblages. Appl Environ Microbiol 70:781–789
Bernhard AE, Landry ZC, Blevins A, José R, Giblin AE, Stahl DA (2010) Abundance of ammonia-oxidizing archaea and bacteria along an estuarine salinity gradient in relation to potential nitrification rates. Appl Environ Microbiol 76:1285–1289
Blaber SJ (2008) Tropical estuarine fishes: ecology, exploration and conservation. Wiley, New York
Bollmann A, Bullerjahn GS, McKay RM (2014) Abundance and diversity of ammonia-oxidizing archaea and bacteria in sediments of trophic end members of the Laurentian Great Lakes, Erie and Superior. PLoS ONE 9:e97068
Boström KH, Simu K, Hagström Å, Riemann L (2004) Optimization of DNA extraction for quantitative marine bacterioplankton community analysis. Limnol Oceanogr 2:365–373
Braak CT, Smilauer P (1998) CANOCO reference manual and user’s guide to Canoco for Windows: software for canonical community ordination (version 4). Centre for Biometry, Wageningen
Brochier-Armanet C, Boussau B, Gribaldo S, Forterre P (2008) Mesophilic Crenarchaeota: proposal for a third archaeal phylum, the Thaumarchaeota. Nat Rev Microbiol 6:245
Caffrey JM, Bano N, Kalanetra K, Hollibaugh JT (2007) Ammonia oxidation and ammonia-oxidizing bacteria and archaea from estuaries with differing histories of hypoxia. ISME J 1:660–662
Clarke K, Warwick R (1994) An approach to statistical analysis and interpretation. Change Mar Communities 2:117–143
Covert JS, Moran MA (2001) Molecular characterization of estuarine bacterial communities that use high- and low-molecular weight fractions of dissolved organic carbon. Aquat Microb Ecol 25:127–139
Cunha M, Almeida M, Alcântara F (2000) Patterns of ectoenzymatic and heterotrophic bacterial activities along a salinity gradient in a shallow tidal estuary. Mar Ecol Prog Ser 204:1–12
De Corte D, Yokokawa T, Varela MM, Agogué H, Herndl GJ (2009) Spatial distribution of Bacteria and Archaea and amoA gene copy numbers throughout the water column of the Eastern Mediterranean Sea. ISME J 3:147–158
DeLong EF, Taylor LT, Marsh TL, Preston CM (1999) Visualization and enumeration of marine planktonic archaea and bacteria by using polyribonucleotide probes and fluorescent in situ hybridization. Appl Environ Microbiol 65:5554–5563
Eilers H, Pernthaler J, Glöckner FO, Amann R (2000) Culturability and in situ abundance of pelagic bacteria from the North Sea. Appl Environ Microbiol 66:3044–3051
Eyre B, Balls P (1999) A comparative study of nutrient behavior along the salinity gradient of tropical and temperate estuaries. Estuaries 22:313–326
Falkowski PG (1997) Evolution of the nitrogen cycle and its influence on the biological sequestration of CO2 in the ocean. Nature 387:272
Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB (2005) Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc Natl Acad Sci U S A 102:14683–14688
Glöckner FO, Fuchs BM, Amann R (1999) Bacterioplankton compositions of lakes and oceans: a first comparison based on fluorescence in situ hybridization. Appl Environ Microbiol 65:3721–3726
Grasshoff K, Ehrhardt M, Kremling K (1983) Methods of seawater analyses. Verlag Chemie 511:342–355
Green S, Leigh M, Neufeld J (2010) Denaturing gradient gel electrophoresis (DGGE) for microbial community analysis. In: Timmis K (ed) Handbook of hydrocarbon and lipid microbiology. Springer, New York, pp 4137–4158
Hou J, Song C, Cao X, Zhou Y (2013) Shifts between ammonia-oxidizing bacteria and archaea in relation to nitrification potential across trophic gradients in two large Chinese lakes (Lake Taihu and Lake Chaohu. Water Res 47:2285–2296
Kemp W, Testa J, Conley D, Gilbert D, Hagy J (2009) Temporal responses of coastal hypoxia to nutrient loading and physical controls. Biogeosciences 6:2985–3008
Könneke M, Bernhard AE, José R, Walker CB, Waterbury JB, Stahl DA (2005) Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437:543–546
Lam P et al (2007) Linking crenarchaeal and bacterial nitrification to anammox in the Black Sea. Proc Natl Acad Sci U S A 104:7104–7109
Li J, Nedwell DB, Beddow J, Dumbrell AJ, McKew BA, Thorpe EL, Whitby C (2015) amoA gene abundances and nitrification potential rates suggest that benthic ammonia-oxidizing bacteria and not archaea dominate N cycling in the Colne Estuary, United Kingdom. Appl Environ Microbiol 81:159–165
Liu S, Shen L, Lou L, Tian G, Zheng P, Hu B (2013) Spatial distribution and factors shaping the niche segregation of ammonia-oxidizing microorganisms in the Qiantang River, China. Appl Environ Microbiol 79:4065–4071
Liu B, Li Y, Zhang J, Zhou X, Wu C (2014) Abundance and diversity of ammonia-oxidizing microorganisms in the sediments of Jinshan Lake. Curr Microbiol 69:751–757
Madhu N et al (2007) Monsoonal impact on planktonic standing stock and abundance in a tropical estuary (Cochin backwaters–India. Estuar Coast Shelf Sci 73:54–64
Martin G et al (2011) Eutrophication induced changes in benthic community structure of a flow-restricted tropical estuary (Cochin backwaters), India. Environ Monit Assess 176:427–438
Menon N, Balchand A, Menon N (2000) Hydrobiology of the Cochin backwater system–a review. Hydrobiologia 430:149–183
Mincer TJ, Church MJ, Taylor LT, Preston C, Karl DM, DeLong EF (2007) Quantitative distribution of presumptive archaeal and bacterial nitrifiers in Monterey Bay and the North Pacific Subtropical Gyre. Environ Microbiol 9:1162–1175
Miranda J, Balachandran K, Ramesh R, Wafar M (2008) Nitrification in Kochi backwaters. Estuar Coast Shelf Sci 78:291–300
Parvathi A et al (2014) Effects of hydrography on the distribution of bacteria and virus in Cochin estuary, India. Ecol Res 30:85–92
Porter KG, Feig YS (1980) The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr 25:943–948
Qasim SZ (2003) Indian estuaries. Allied Publishers, New Delhi
Quan X, Wang Y, Xiong W, He M, Yang Z, Lin C (2010) Description of microbial community structure of sediments from the Daliao River water system and its estuary (NE China) by application of fluorescence in situ hybridization. Environ Earth Sci 61:1725–1734
Ram AP, Nair S, Chandramohan D (2007) Bacterial growth efficiency in a tropical estuary: seasonal variability subsidized by allochthonous carbon. Microb Ecol 53:591–599
Rappé MS, Giovannoni SJ (2003) The uncultured microbial majority. Annu Rev Microbiol 57:369–394
Sahan E, Muyzer G (2008) Diversity and spatio-temporal distribution of ammonia-oxidizing Archaea and Bacteria in sediments of the Westerschelde estuary. FEMS Microbiol Ecol 64:175–186
Sarma V et al (2011) High CO2 emissions from the tropical Godavari estuary (India) associated with monsoon river discharges. Geophys Res Lett 38
Seitzinger SP (1988) Denitrification in freshwater and coastal marine ecosystems: ecological and geochemical significance. Limnol Oceanogr 33:702–724
Sheeba V, Abdulaziz A, Gireeshkumar T, Ram A, Rakesh P, Jasmin C, Parameswaran P (2017) Role of heavy metals in structuring the microbial community associated with particulate matter in a tropical estuary. Environ Pollut 231:589–600
Shish FK, Ducklow HW (1994) Temperature regulation of heterotrophic bacterioplankton abundance, production, and specific growth rate in Chesapeake Bay. Limnol Oceanogr 39:1243–1258
Singh SK, Verma P, Ramaiah N, Chandrashekar AA, Shouche YS (2010) Phylogenetic diversity of archaeal 16S rRNA and ammonia monooxygenase genes from tropical estuarine sediments on the central west coast of India. Res Microbiol 161:177–186
Stahl D (1991) Development and application of nucleic acid probes. Wiley, New York, pp 205–248
Tai X et al (2013) High diversity of nitrogen-fixing bacteria in the upper reaches of the Heihe River, northwestern China. Biogeosciences 10:5589–5600
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Thingstad F, Billen G (1994) Microbial degradation of Phaeocystis material in the water column. J Mar Syst 5:55–65
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl Acids Res 25:4876–4882
Thottathil SD, Balachandran K, Gupta G, Madhu N, Nair S (2008) Influence of allochthonous input on autotrophic–heterotrophic switch-over in shallow waters of a tropical estuary (Cochin Estuary), India. Estuar Coast Shelf Sci 78:551–562
Vinita J, Shivaprasad A, Revichandran C, Manoj N, Muraleedharan K, Binzy J (2015) Salinity response to seasonal runoff in a complex estuarine system (Cochin Estuary, West Coast of India). J Coast Res 31:869
Vipindas et al (2015) Bacterial domination over Archaea in ammonia oxidation in a monsoon-driven tropical estuary. Microb Ecol 69:544–553
Vipindas P, Anas A, Jayalakshmy K, Lallu K, Benny P, Shanta N (2018) Impact of seasonal changes in nutrient loading on distribution and activity of nitrifiers in a tropical estuary. Cont Shelf Res 154:37–45
Voss M et al (2011) History and scenarios of future development of Baltic Sea eutrophication. Estuar Coast Shelf Sci 92:307–322
Wang X, Wang C, Bao L, Xie S (2014) Abundance and community structure of ammonia-oxidizing microorganisms in reservoir sediment and adjacent soils. Appl Microbiol Biotechnol 98:1883–1892
Wankel SD, Mosier AC, Hansel CM, Paytan A, Francis CA (2011) Spatial variability in nitrification rates and ammonia-oxidizing microbial communities in the agriculturally impacted Elkhorn Slough estuary, California. Appl Environ Microbiol 77:269–280
Wikner J, Cuadros R, Jansson M (1999) Differences in consumption of allochthonous DOC under limnic and estuarine conditions in a watershed. Aquat Microb Ecol 17:289–299
Wuchter C et al (2006) Archaeal nitrification in the ocean. Proc Natl Acad Sci U S A 103:12317–12322
Zhang Q, Tang F, Zhou Y, Xu J, Chen H, Wang M, Laanbroek HJ (2015) Shifts in the pelagic ammonia-oxidizing microbial communities along the eutrophic estuary of Yong River in Ningbo City, China. Front Microbiol 6:1–15
Zheng Y et al (2014) Community dynamics and activity of ammonia-oxidizing prokaryotes in intertidal sediments of the Yangtze Estuary. Appl Environ Microbiol 80:408–419
Acknowledgements
We are thankful to the Director, CSIR-National Institute of Oceanography (NIO), Goa, the Scientist-in-charge, CSIR-NIO, RC-Kochi and the Head, Department of Marine Biology, Microbiology and Biochemistry, Cochin University of Science and Technology for providing necessary facilities to carry out the work. The authors are grateful to COMAPS-ICMAM project and Centre for Marine Living Resources and Ecology, Ministry of Earth Sciences, Govt. of India for financial and technical support. The authors express their gratitude to Dr. C.T. Achuthankutty, Chief Scientist (Retired), CSIR-National Institute of Oceanography, Goa for critically reading the manuscript and improving its presentation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vipindas, P.V., Jabir, T., Jasmin, C. et al. Diversity and seasonal distribution of ammonia-oxidizing archaea in the water column of a tropical estuary along the southeast Arabian Sea. World J Microbiol Biotechnol 34, 188 (2018). https://doi.org/10.1007/s11274-018-2570-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-018-2570-0