Abstract
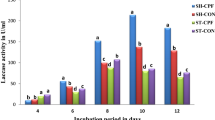
Nine aromatic compounds (caffeic acid, syringaldehyde, vanillic acid, guaiacol, vanillin, sinapic acid, syringol, syringic acid and ferulic acid) and four metallic compounds (CuSO4, AgNO3, MnSO4, and CaCl2) were tested for their ability to increase laccase (Lac) activity in the ligninolytic basidiomycete Phlebia brevispora BAFC 633. The addition of syringaldehyde, syringol, guaiacol, sinapic acid, vanillin, ferulic acid and CuSO4 showed a positive effect on fungal growth; however, it decreased dramatically with the addition of AgNO3 and did not undergo changes in the presence of CaCl2 or MnSO4. Lac activity increased with the addition of all the compounds tested, depending on the concentration and the day of culture. P. brevispora BAFC 633 produced two isoenzymes, a constitutively expressed of 60 kDa and another of 75 kDa expressed upon induction by sinapic acid, MnSO4 or CuSO4. Lac secretion capacity of P. brevispora BAFC 633 can be increased 27 times higher than the control with the highest levels detected in the presence of 0.3 mM CuSO4 at day 14. The action is affected at pre-transcriptional level regulating at the onset of the process, however it does not rule out the effect at the post-transcriptional and post-translational levels, for which is necessary to deepen in the knowledge of all possible regulation points of gene expression.







Similar content being viewed by others
References
Baldrian P (2003) Interactions of heavy metals with white-rot fungi. Enzym Microb Technol 32:78–91
Bollag JM, Leonowicz A (1984) Comparative studies of extracellular fungal laccases. Appl Environ Microbiol 48:849–854
Buswell JM, Odier E (1987) Lignin biodegradation. CRC Crit Rev Biotechnol 6:1–60
Cavallazzi JRP, KasuyaI CM, Soares MA (2005) Screening of inducers for laccase production by Lentinula edodes in liquid médium. Braz J Microbiol 36:383–387
Chen CL, Chanoh M, Kirk TK (1982) Aromatic acids produced during degradation of lignin in spruce wood by Phanerochaete chrysosporim. Holzforschung 36:3–9
Cho N-S, Kim D-H, Cho H-Y, Ohga S, Leonowicz A (2006) Effect of various compounds on the activity of laccases from basidiomycetes and their oxidative and demethoxylating activities. J Fac Agr Kyushu U 51:211–218
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Collins PJ, Dobson A (2002) Regulation of laccase gene transcription in Trametes versicolor. Enzym Microb Technol 31:3–9
D´Souza TM, Merritt CS, Reddy CA (1999) Lignin modifying enzymes of the white rot basidiomycete Ganoderma lucidum. Appl Environ Microb 65:5307–5313
D´Souza-Ticlo DT (2008) The lignin-degrading enzyme, laccase from marine fungi; biochemical and molecular approaches. Thesis doctoral, Goa University, Dona Paula, Goa, India
D’Souza TM, Boominathan K, Reddy CA (1996) Isolation of laccase gene-specific sequences from white rot and brown rot fungi by PCR. Appl Environ Microb 62:3739–3744
De la Rubia T, Linares A, Pérez J, Muňoz-Dorado J, Romera J, Martínez J (2002) Characterization of manganese-dependent peroxidase isoenzymes from the ligninolytic fungus Phanerochaete flavido alba. Res Microbiol 153:547–554
Eggert C, Temp U, Eriksson E (1996) The ligninolytic system of the white rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl Environ Microb 62:1151–1158
Elisashvili V, Kachlishvili E, Tsiklauri N, Metreveli E, Khardziani T, Agathos SN (2009) Lignocellulose degrading enzyme production by white-rot Basidiomycetes isolated from the forests of Georgia. World J Microb Biotechnol 25:331–339
Faraco V, Giardina P, Palmieri G, Sannia G (2002) Metal-activated laccase promoters. Biotechnol Progr 21:105–111
Faraco V, Giardina P, Sannia G (2003) Metal-responsive elements in Pleurotus ostreatus laccase gene promoters. Microbiology 149:2155–2162
Fernández-Larrea J, Stahl U (1996) Isolation and characterization of a laccase gene from Podospora anserina. Mol Genet Genomics 252:539–551
Field JA, Jong E, Feijoo-Costa G, Bont JAM (1993) Screening for ligninolytic fungi applicable to the biodegradation of xenobiotics. Trends Biotechnol 11:44–49
Fonseca MI (2012) Utilización de hongos de pudrición blanca de la Provincia de Misiones en procesos de biopulpado: aspectos bioquímicos y moleculares de sistemas ligninolíticos involucrados y prospección biotecnológica. Tesis Doctoral, Universidad Nacional de Tucumán
Fonseca MI, Shimizu E, Villalba LL, Zapata PD (2010) Laccase-producing ability and the inducing effect of Copper on laccase production of white rot fungi native from Misiones (Argentina). Enzym Microb Technol 46:534–539
Fonseca MI, Fariña JI, Sanabria NI et al (2013) Influence of culture conditions on laccase production, growth, and isoenzymes patterns in native white rot fungi from the misiones rainforest (Argentina). BioResources 8:2855–2866
Gadd GM (1993) Interactions of fungi with toxic metals. New Phytol 124:25–60
Galhaup C, Haltrich D (2001) Enhanced formation of laccase activity by the white-rot fungus Trametes pubescens in the presence of copper. Appl Microbiol Biotechnol 56:225–232
Galhaup C, Goller S, Peterbauer K et al (2003) Characterization of the major laccase isoenzyme from Trametes pubescens and regulation of its synthesis by metal ions. Microbiology 148:2159–2169
Gorbatova ON, Koroleva OV, Landesman EO et al (2006) Increase of the detoxification potential of basidiomycetes by induction of laccase biosynthesis. Appl Biochem Microbiol 42:414–419
Guillén Y, Machuca A (2008) The effect of copper on the growth of wood-rotting fungi and a blue-stain fungus. World J Micro Biot 24:31–37
Horikoshi T, Nakajima A, Sakaguchi T (1981) Studies on the accumulation of heavy metal elements in biological systems: accumulation of uranium by microorganisms. Eur J Appl Microbiol Biotechnol 12:90–96
Ikehata K, Buchanan ID, Smith DW (2004) Recent development in the production of extracellular fungal peroxidases and laccases for waste treatment. J Environ Eng Sci 3:1–19
Jellison J, Connolly J, Goodell B (1997) The role of cations in the biodegradation of wood by the brown rot fungi. Int Biodeterior Biodegrad 39:165–179
Kirk TK, Farrell RL (1987) Enzymatic “combustion”: the microbial degradation of lignin. Annu Rev Microbiol 41:465–505
KoroIjova-Skorobogat’ko OV, Stepanova EV, Gavrilova V et al (1998) Purification and characterization of the constitutive form of laccase from the basidiomycete Coriolus hirsutus and effect of inducers on laccase synthesis. Biotechnol Appl Biochem 28:47–54
Kubicek CP, Hampel WA, Röhr M (1979) Manganese deficiency leads to elevated amino acid pool levels in citric acid accumulating Aspergillus niger. Arch Microbiol 123:73–79
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lee IY, Jung KH, Lee CH, Park YH (1999) Enhanced production of laccase in Trametes versicolor by the addition of ethanol. Biotechnol Lett 21:965–968
Levin L, Forchiassin F, Ramos AM (2002) Copper induction of lignin modifying enzymes in the white rot fungus Trametes trogii. Mycologia 94:377–383
Lu X, Ding S (2010) Effect of Cu2+, Mn2+ and aromatic compounds on the production of laccase isoforms by Coprinus comatus. Mycoscience 51:68–74
Luis P, Walther G, Kellner H, Martin F, Buscot F (2004) Diversity of laccase genes from basidiomycetes in a forest soil. Soil Biol Biochem 36:1025–1036
Mäkelä MR, Hildén KS, Hakala TK, Hatakka A, Lundell TK (2006) Expression and molecular properties of a new laccase of the white rot fungus Phlebia radiata grown on wood. Curr Genet 50:323–333
Mansur M, Suárez T, González AE (1998) Differential gene expression in the laccase gene family from Basidiomycete I–62 (CECT 20197). Appl Environ Microbiol 64:771–774
Mayer AM, Stapler RC (2002) Laccase: new functions for an old enzyme. Phytochemistry 60:551–566
Minussi RC, Pastore GM, Durán N (2002) Potential applications of laccase in the food industry. Trends Food Sci Technol 13:205–216
Muñoz C, Guillen F, Martinez AT, Martinez MJ (1997) Induction and characterization of laccase in the ligninolytic fungus Pleurotus eryngii. Curr Microbiol 34:1–5
Murugesan K, Nam IH, Kim YM, Chang YS (2007) Decolorization of reactive dyes by a thermostable laccase produced by Ganoderma lucidum in solid culture. Enzym Microbial Technol 40:1662–1672
Murugesan K, Chang YY, Kim YM et al (2010) Enhanced transformation of triclosan by laccase in the presence of redox mediators. Water Res 44:298–308
Myasoedova NM, Chernykh AM, Psurtseva NV et al (2008) New efficient producers of fungal laccases. Appl Microbiol Biotechnol 44:73–77
Orthofer R, Kubicek CP, Rohr M (1979) Lipid levels and manganese deficiency in various citric acid producing strains of Aspergillus niger. FEMS Microbiol Lett 5:403–406
Palmieri G, Giardina P, Bianco C et al (2000) Copper induction of laccase isoenzymes in the ligninolytic fungus Pleurotus ostreatus. Appl Environ Microbiol 66:920–924
Patel H, Gupte A, Gupte S (2009) Effect of different culture conditions and inducers on production of laccase by Basidiomycetes fungal isolate Pleurotus ostreatus HP-1 under solid state fermentation. BioResources 4:268–284
Pezzella C, Autore F, Giardina P et al (2009) The Pleurotus ostreatus laccase multi-gene family: isolation and heterologous expression of new family members. Curr Genet 55:45–57
Piscitelli A, Giardina P, Lettera V, Pezzella C, Sannia G, Faraco V (2011) Induction and transcriptional regulation of laccases in fungi. Curr Genomics 12:104–112
Preussler CA, Shimizu E, Villalba LL, Zapata PD (2009) Inducción con cobre de la enzima lacasa en el hongo de pudrición blanca Trametes villosa (Sw.:Fr.) Kreisel. Rev Cien Tecnol 12:9–16
Rollins JA, Dickman MB (1998) Increase in endogenous and exogenous cyclic AMP levels inhibits sclerotial development in Sclerotinia sclerotiorum. Appl Environ Microbiol 64:2539–2544
Ryan S, Schnitzhofer W, Tzanov T et al (2003) An acid-stable laccase from Sclerotium rolfsii with potential for wool dye decolourization. Enzym Microb Technol 33:766–774
Salony MS, Bisaria VS (2006) Production and characterization of laccase from Cyathus bulleri and its use in decolourization of recalcitrant textile dyes. Appl Microbiol Biotechnol 71:646–653
Saparrat M, Balatti PA, Martínez MJ, Jurado M (2010) Differential regulation of laccase gene expression in Coriolopsis rigida LPSC no. 232. Fungal Biol 114:999–1006
Soden DM, Dobson ADW (2001) Differential regulation of laccase gene expression in Pleurotus sajorcaju. Microbiology 147:1755–1763
Stajic M, Persky L, Hadar Y, Friesem D, Duletic-Lausevic S, Wasser SP, Nevo E (2006) Effect of copper and manganese ions on activities of laccase and peroxidases in three Pleurotus species grown on agricultural wastes. Appl Biochem Biotechnol 128:87–96
Terron MC, Gonzalez T, Carbajo JM et al (2004) Structurally closely-related aromatic compounds have different effects on laccase activity and on lcc gene expression in the ligninolytic fungus Trametes sp. I-62. Fungal Genet Biol 41:954–962
Thurston CF (1994) The structure and function of fungal laccases. Microbiology 140:19–26
Xiao YZ, Tu XM, Wang J et al (2003) Purification, molecular characterization and reactivity with aromatic compounds of a laccase from basidiomicete Trametes sp. strain AH28-2. Appl Microbiol Biotechnol 60:700–707
Xiao YZ, Chen Q, Wu J et al (2004) Selective induction, purification and characterization of a laccase isozyme from the basidiomycete Trametes sp. AH28-2. Mycologia 96:26–35
Xiao YZ, Hong YZ, Li JF, Hang J, Tong PG, Fang W, Zhou CZ (2006) Cloning of novel laccase isozyme genes from Trametes sp. AH28-2 and analyses of their differential expression. Appl Microbiol Biotechnol 71:493–501
Yaver DS, Golightly EJ (1996) Cloning and characterization of three laccase genes from the white-rot basidiomycete Trametes villosa: genomic organization of the laccase gene family. Gene 181:95–102
Youn HD, Hah YC, Kang SO (1995) Role of laccase in lignin degradation by white-rot fungi. FEMS Microbiol Lett 132:183–188
Acknowledgments
Authors wish to thank financial support from Secretaría de Ciencia y Tecnología de la Universidad Nacional de Misiones, through grants for innovation projects (16Q446 and 16Q486). MIF and SSSA have a fellowship for doctoral studies from CONICET, Argentina.
Conflict of interest
All authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fonseca, M.I., Ramos-Hryb, A.B., Fariña, J.I. et al. Effect of chemical and metallic compounds on biomass, mRNA levels and laccase activity of Phlebia brevispora BAFC 633. World J Microbiol Biotechnol 30, 2251–2262 (2014). https://doi.org/10.1007/s11274-014-1646-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-014-1646-8