Abstract
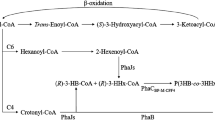
Ecological deterioration and human health concerns arising from the usage of non-biodegradable plastics have prompted mankind to search for greener alternatives which are biodegradable, biocompatible and easily produced from renewable sources. Polyhydroxyalkanoates (PHA), among other biopolymers, are emerging as a viable replacement for fossil fuel-based synthetic plastics. A PHA-producing strain, identified as Cupriavidus sp. (designated Cupriavidus sp. USMAA2-4) was isolated from a soil sample from western peninsular Malaysia. Heterologous expression of the PHA synthase gene (phaC USMAA2-4) in mutant C. necator PHB−4 complemented its PHA-producing ability. More than 60 wt% of P(3HB) was synthesized from various plant oils. The highest P(3HB) production of 2.38 g/l at 68 wt% was attained when crude palm kernel oil was fed as the sole carbon source. The 3HV molar fraction in poly(3-hydroxybutyrate-co-3-hydroxyvalerate) [P(3HB-co-3HV)] was significantly affected by the type of the precursor used and their respective feeding time. The 3HV molar fraction ranged from 4 to 31 mol% when sodium propionate/valerate was fed at different cultivation times. In addition, with the supplementation of 4HB-monomer precursors, approximately 67 wt% P(3HB-co-4HB) with 4–5 mol% of 4-hydroxybutyrate monomer was synthesized, regardless of the precursor feeding time used. Variation in the molar fraction of the second monomer along with its biodegradability and biocompatibility characteristics promotes the potential of these copolymers as replacements for traditional commodity plastics.



Similar content being viewed by others
References
Akiyama M, Tsuge T, Doi Y (2003) Environmental life cycle comparison of polyhydroxyalkanoates produced from renewable resources by bacterial fermentation. Polym Degrad Stabil 80:183–194
Amirul AAA, Tay BY, Chang CW, Azizan MNM, Majid MIA, Sudesh K (2004) Biosynthesis and characterization of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) produced by Ralstonia sp. USMAA2-4 isolated from soil. J Biosains 15:125–135
Ashby RD, Solaiman DKY (2008) Poly(hydroxyalkanoate) biosynthesis from crude Alaskan Pollock (Theragra chalcogramma) oil. J Environ Polym Degr 16:221–229
Braunegg G, Sonnleitner B, Lafferty RM (1978) A rapid gas chromatographic method for the determination of poly-β-hydroxybutyric acid in microbial biomass. Eur J Microbiol Biotechnol 6:29–37
Byrom D (1997) Polymer synthesis by microorganisms; technology and economics. Trends Biotechnol 5:246–250
Chen GQ, Wu Q (2005) The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 26:6565–6578
Dionisi D, Carucci G, Papini MP, Majone M, Carrassco F (2005) Olive oil mill effluents as a feedstock for production of biodegradable polymers. Water Res 39:2076–2084
Doi Y, Kunioka M, Nakamura Y, Soga K (1987) Biosynthesis of copolyesters in Alcaligenes eutrophus H16 from 13C-labeled acetate and propionate. Macromolecules 20:2988–2991
Doi Y, Kitamura S, Abe H (1995) Microbial synthesis and characterization of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Macromolecules 28:4822–4828
Du GC, Chen J, Yu J, Lun SY (2001) Feeding strategy of propionic acid for production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with Ralstonia eutropha. Biochem Eng J 8:103–110
Friedrich B, Hogrefe C, Schlegel HG (1981) Naturally occurring genetic transfer of hydrogen-oxidizing ability between strains of Alcaligenes eutrophus. J Bacteriol 147:198–205
Fukui T, Doi Y (1997) Cloning and analysis of the poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) biosynthesis genes of Aeromonas caviae. J Bacteriol 179:4821–4830
Grengross TU, Slater S (2000) How green are green plastics? Sci Am 8:37–41
Hermawan S, Jendrossek D (2007) Microscopial investigation of poly(3-hydroxybutyrate) granule formation in Azotobacter vinelandii. FEMS Microbiol Lett 266:60–64
Jendrossek D (2005) Fluorescence microscopical investigation of poly(3-hydroxybutyrate) granule formation in bacteria. Biomacromolecules 6:598–603
Jendrossek D, Selchow O, Hoppert M (2007) PHB granules at the early stages of formation are localized close to the cytoplasmic membrane in Caryophanon latum. Appl Environ Microbiol 73:586–593
Keenan TM, Nakas JP, Tanenbaum SW (2006) Polyhydroxyalkanoate copolymers from forest biomass. World J Microbiol Biotechnol 33:616–626
Kek YK, Lee WH, Sudesh K (2008) Efficient bioconversion of palm acid oil and palm kernel acid oil to poly(3-hydroxybutyrate) by Cupriavidus necator. Can J Chem 86:533–539
Khanna S, Srivastava AK (2007) Production of poly(3-hydroxybutyric-co-3-hydroxyvaleric acid) having a high hydroxyvalerate content with valeric acid feeding. J Ind Microbiol Biotechnol 34:457–461
Kim BS, Lee SY, Chang HN, Chang YK, Woo SI (1994) Production of poly(3-hydroxybutyric-co-3-hydroxyvaleric acid) by fed-batch culture of Alcaligenes eutrophus with substrate control using on-line glucose analyzer. Enzyme Microbiol Technol 16:556–561
Koller M, Bona R, Braunegg G, Hermann C, Horvat P, Kroutil M, Martinz J (2005) Production of polyhydroxyalkanoates from agricultural waste and surplus materials. Biomacromolecules 6:561–565
Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM, Peterson KM (1995) Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176
Lee WH, Azizan MNM, Sudesh K (2004) Effects of culture conditions of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) synthesized by Comamonas acidovorans. Polym Degrad Stab 84:129–134
Lee WH, Loo CY, Nomura CT, Sudesh K (2008) Biosynthesis of polyhydroxyalkanoate copolymers from mixtures of plant oils and 3-hydroxyvalerate precursors. Bioresour Technol 99:6844–6851
Loo CY, Sudesh K (2007) Biosynthesis and native granule characteristics of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in Delftia acidovorans. Int J Biol Macromol 40:466–471
Loo CY, Lee WH, Tsuge T, Doi Y, Sudesh K (2005) Biosynthesis and characterization of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from palm oil products in a Wautersia eutropha mutant. Biotechnol Lett 27:1405–1410
López-Cortés A, Rodríguez-Fernández O, Latisnere-Barragán H, Mejía-Ruíz HC, González-Gutiérrez G, Lomelí-Ortega C (2010) Characterization of polyhydroxyalkanoate and the phaC gene of Paracoccus seriniphilus E71 strain isolated from a polluted marine microbial mat. World J Microbiol Biotechnol 26:109–118
Madison LL, Huisman GW (1999) Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol Mol Biol R 63:21–53
McDowell EM, Trump BF (1976) Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med 100:405–414
Mohanty AK, Misra M, Drzal LT (2002) Sustainable bio-composites from renewable resources: opportunities and challenges in the green materials world. J Environ Polym Degr 10:19–26
Naik S, Gopal SKV, Somal P (2008) Bioproduction of polyhydroxyalkanoates from bacteria: a metabolic approach. World J Microbiol Biotechnol 24:2307–2314
Narodoslawsky M, Niederl-Schmidinger A, Halasz L (2008) Utilising renewable resourses economically: new challenges and chances for process development. J Clean Prod 16:164–170
Nomura C, Taguchi S (2007) PHA synthase engineering toward superbiocatalysts for custom-made biopolymers. Appl Microbiol Biotechnol 73:969–979
Peters V, Becher D, Rehm BHA (2007) The inherent property of polyhydroxyalkanoate synthase to form spherical PHA granules at the cell poles: the core region is required for polar localization. J Biotechnol 132:238–245
Philip S, Keshavarz T, Roy I (2007) Polyhydroxyalkanoates: biodegradable polymers with a range of applications. J Chem Technol Biotechnol 82:233–247
Prabhu NN, Santimano MC, Mavinkurve S, Bhosle SN, Garg S (2010) Native granule associated short chain length polyhydroxyalkanoate synthase from a marine derived Bacillus sp. NQ-11/A2. Anton Leeuw 97:41–50
Rahayu A, Zaleha Z, Yahya ARM, Majid MIA, Amirul AA (2008) Production of copolymer poly(3-hydroxybutyrate-co-4-hydroxybutyrate) through a one-step cultivation process. World J Microbiol Biotechnol 24:2403–2409
Reddy SV, Thirumala M, Reddy TVK, Mahmood SK (2008) Isolation of bacteria producing polyhydroxyalkanoates (PHA) from municipal sewage sludge. World J Microbiol Biotechnol 24:2949–2955
Rehm BHA (2003) Polyester synthases: natural catalysts for plastics. Biochem J 376:15–33
Rehm BHA (2007) Biogenesis of microbial polyhydroxyalkanoate granules: a platform technology for the production of tailor-made bioparticles. Curr Issues Mol Biol 9:41–62
Rehm BHA, Steinbüchel A (1999) Biochemical and genetic analysis of PHA synthases and other proteins required for PHA synthesis. Int J Biol Macromol 13:83–88
Rehm BHA, Steinbüchel A (2001) PHA synthases: key enzymes of PHA biosynthesis. In: Steinbüchel A, Doi Y (eds) Biopolymers polyesters I, 3a. Wiley-VCH, Weinheim, pp 173–215
Rehm BHA, Steinbüchel A (2002) PHA synthases: the key enzymes of PHA synthesis. In: Doi Y, Steinbüchel A (eds) Biopolymers, vol 3a. Wiley/VCH, Weinheim, pp 173–215
Rocha RCS, da Silva LF, Taciro MK, Pradella JGC (2008) Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) P(3HB-co-3HV) with a broad range of 3HV content at high yields by Burkholderia sacchari IPT 189. World J Microbiol Biotechnol 24:427–431
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Simon R, Priefer U, Pühler A (1983) A broad host range mobilization system for In vivo genetic engineering. Transposon mutagenesis in Gram negative bacteria. Biotechnology 1:784–791
Solaiman DKY, Ashby RD, Foglia TA, Marmer WN (2006) Conversion of agricultural feedstock and coproducts into poly(hydroxyalkanoates). Appl Microbiol Biotechnol 71:783–789
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Steinbüchel A, Hein S (2001) Biochemical and molecular basis of microbial synthesis of polyhydroxyalkanoates in microorganisms. Adv Biochem Eng 71:81–123
Stubbe J, Tian JM (2003) Polyhydroxyalkanoate (PHA) homeostasis: the role of the PHA synthase. Nat Prod Rep 20:445–457
Sudesh K, Abe H, Doi Y (2000) Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog Polym Sci 25:1503–1555
Suriyamongkol P, Weselake R, Narine S, Moloney M, Shah S (2007) Biotechnological approaches for the production of polyhydroxyalkanoates in microorganisms and plants—a review. Biotechnol Adv 25:148–175
Tay BY, Lokesh BE, Lee CY, Sudesh K (2009) Polyhydroxyalkanoate (PHA) accumulating bacteria from the gut of higher termite Macrotermes carbonarius (Blattodea: Termitidae). World J Microbiol Biotechnol. doi:10.1007/s11274-009-0264-3
Tian J, Sinskey AJ, Stubbe J (2005) Kinetic studies of polyhydroxyalkanoate granule formation in Wautersia eutropha H16 by transmission electron microscopy. J Bacteriol 187:3814–3824
Tsuge T (2002) Metabolic improvements and use of inexpensive carbon sources in microbial production of polyhydroxyalkanoates. J Biosci Bioeng 94:579–584
Verlinden RAJ, Hill DJ, Kenward MA, Williams CD, Radecka I (2007) Bacterial synthesis of biodegradable polyhydroxyalkanoates. J Appl Microbiol 102:1437–1449
Vigneswari S, Nik LA, Majid MIA, Amirul AA (2009) Improved production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) copolymer using a combination of 1,4-butanediol and γ-butyrolactone. World J Microbiol Biotechnol. doi:10.1007/s11274-009-0207-z
Wodzinska J, Snell KD, Rhomberg A, Sinskey AJ, Biemann K, Stubbe J (1996) Polyhydroxybutyrate synthase: evidence for covalent catalysis. J Am Chem Soc 118:6319–6320
Acknowledgments
We thank Dr. M. E. Kovach for the pBBR1MCS-2 vector used in this study. We are grateful to Professor Dr. John Leslie for editing this manuscript. This work was supported by a SAGA grant 304/PBIOLOGI/653009/A118 from the Academy of Sciences Malaysia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kek, YK., Chang, CW., Amirul, AA. et al. Heterologous expression of Cupriavidus sp. USMAA2-4 PHA synthase gene in PHB−4 mutant for the production of poly(3-hydroxybutyrate) and its copolymers. World J Microbiol Biotechnol 26, 1595–1603 (2010). https://doi.org/10.1007/s11274-010-0335-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-010-0335-5