Abstract
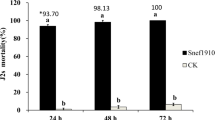
Root-knot nematodes are serious pathogens that severe damage to major crops. They damage plant root system that caused significant yield losses. Moreover, the predisposition of nematode-infected plants is secondary infection from fungal plant pathogen that additional adverse effects on plant growth. Our target is to find the antagonist for control nematode, and secondary infection agents and stimulate plant growth. Twenty-three plant-parasitic nematode infested soils were taken from some provinces in the northern and center of Thailand and actinomycetes and fungi were isolated. Eighty-three isolates belong to actinomycete and 67 isolates were fungi. The predominant actinomycete taxa was Streptomyces (97.6%). The predominant fungal taxa were Penicillium (37.3%) and Fusarium (32.8%). All actinomycete and fungal isolates were subjected for primary screening in vitro for their effects on egg hatching and juvenile mortality of Meloidogyne incognita. Secondary screening was evaluated for antagonist effect on plant pathogenic fungi collected from nematode-infected plant, plant growth hormone (indole-3-acetic acid; IAA) and siderophore production. From primary screening, 7 actinomycete and 10 fungal isolates reduced egg hatching and kill juveniles of M. incognita after 7 days incubation. In secondary screening, 10 nematophagous microbes produced IAA and 9 isolates produced hydroxamate siderophore. Streptomyces sp. CMU-MH021 was selected as a potential biocontrol agent. It reduced egg hatching rate to 33.1% and increased juvenile mortality rate to 82% as contrasted to the control of 79.6 and 3.6%, respectively. This strain had high activity to against tested fungi and high ability on IAA (28.5 μg ml−1) and siderophore (26.0 μg ml−1) production.




Similar content being viewed by others
Abbreviations
- Ca:
-
Circa
- CAS:
-
Chrome azurol S
- RNKs:
-
Root-knot nematodes
References
Arnow LE (1937) Colorimetric estimation of the components of 3, 4-dihydroxy phenylalanine tyrosine mixtures. J Biol Chem 118:531–535
Atalan E, Manfio GP, Ward AC, Kroppenstedt RM, Goodfellow M (2000) Biosystematic studies on novel Streptomyces from soil. Antonie Van Leeuwenhoek 77:337–353
Bano N, Musarrat J (2003) Characterization of a new Pseudomonas aeruginosa strain NJ-15 as a potential biocontrol agent. Curr Microbiol 46:324–328
Barnett HL, Hunter BB (1987) Illustrated genera of imperfect fungi, 4th edn. Macmillan, New York
Bric JM, Bostock RM, Silversone SE (1991) Rapid in situ assay for indole acetic acid production by bacteria immobilization on a nitrocellulose membrane. Appl Environ Microbiol 57:535–538
Chaiharn M, Chunhaleuchanon S, Lumyong S (2009) Screening siderophore producing bacteria as potential biological control agent for fungal rice pathogens in Thailand. World J Microbiol Biotechnol 25:1919–1928
Chen SY, Dickson DW, Whitty EB (1996) Fungi associated with egg masses of Meloidogyne incognita and M. javanica in a Florida tobacco Weld. Nematropica 26:153–157
Chung KR, Shilts T, Erturk U, Timmer LW, Ueng PP (2003) Indole derivatives produced by the fungus Colletotrichum acutatum causing lime anthracnose and post bloom fruit drop of citrus. FEMS Microbiol Letts 226:23–30
Cliff GM, Hirschmann H (1984) Meloidogyne microcephala n. sp. (Meloidogynidae) a root-knot nematode from Thailand. J Nematol 16:183–193
Csaky T (1948) On the estimation of bound hydroxylamine. Acta Chem Scand 2:450–454
Dicklow MB, Acosta N, Zuckerman BM (1993) A novel Streptomyces species for controlling plant- parasitic nematodes. J Chem Ecol 19:159–173
Dong LQ, Zhang KQ (2006) Microbial control of plant-parasitic nematodes: a five-party interaction. Plant Soil 288:31–45
Dowling DN, O’Gara F (1994) Metabolites of Pseudomonas involved in biocontrol of plant diseases. Trends Biotechnol 12:133–141
El-Nagdi WMA, Youssef MMA (2004) Soaking faba bean seed in some bio-agents as prophylactic treatment for controlling Meloidogyne incognita root-knot nematode infection. J Pest Sci 77:75–78
Esnard J, Potter TL, Zuckerman BM (1995) Streptomyces costaricanus sp. nov., isolated from nematode-suppressive soil. Int J Syst Bacteriol 45:775–779
Godoy G, Rodriguez-kabana R, Morgan-jones G (1983) Fungal parasites of greenhouse studies. Nematropica 13:201–213
Gotlieb D, Oka Y, Ben-Daniel B-H, Cohen Y (2003) Dry mycelium of Penicillium chrysogenum protects cucumber and tomato plants against the root-knot nematode Meloidogyne javanica. Pathoparositica 31:217–225
Gupta CP, Dubey RC, Maheshwari DK (2002) Plant growth enhancement and suppression Macrophomina phaseolina causing charcoal rot of peanut by fluorescent Pseudomonas. Biol Fertil Soils 35:399–405
Handoo ZA, Skantar AM, Carta LA, Erbe EF (2005) Morphological and molecular characterization of a new root-knot nematode, Meloidogyne thailandica n. sp. (Nematoda: Meloidogynidae), parasitizing ginger (Zingiber sp.). J Nematol 37:343–353
Hasegawa T, Takisawa M, Tanida S (1983) A rapid analysis for chemical grouping of aerobic actinomycetes. J Gen Appl Microbiol 29:319–322
Hass D, Defago G (2005) Biological control of soil borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol 3:307–319
He SY, Ge QX (1987) The mycoXora of cotton root-knot nematode (Meloidogyne incognita). Acta Phytopathol Sin 17:14–21
Holt JA, Krieg NR, Sneath PHA (1994) Bergey’s manual of determonative bcteriology. Williams & Wilkins, Baltimore
Hussey RS, Barker KR (1973) A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis Rep 57:1025–1028
Janowicz K, Mazurkiewicz-Zapaowicz K, Wronkowska H (2001) Effect of coexistence of Penicillium frequentans (westling) with Globodera rostochiensis (Woll.) behrens on potato. Arch Phytopathol Plant Prot 34:55–65
Jayakumar J (2009) Streptomyces avermitilis as a biopesticide for the management of root knot nematode, Meloidogyne incognita in tomato. Karnataka J Agric Sci 2:564–566
Jayasinghe BATD, Parkinson D (2007) Actinomycetes as antagonists of litter decomposer fungi. Appl Soil Ecol 38:109–118
Johnson CS (1998) Tobacco. In: Barker KR, Pederson GA, Windham GL (eds) Plant and nematode interactions. American Society of Agronomists, Madison, pp 487–521
Kaewchai S, Soytong K, Hyde KD (2009) Mycofungicides and fungal biofertilizers. Fungal Divers 38:25–50
Khamna S, Yokota A, Lumyong S (2009) Actinomycetes isolated from medicinal plant rhizosphere soils: diversity and screening of antifungal compounds, indole-3-acetic acid and siderophore production. World J Microbiol Biotechnol 25:649–655
Kravchenko LV, Azarova TS, Makarova NM, Tikhonovich IA (2004) The effect of tryptophan present in plant root exudates on the phytostimulating activity of rhizobacteria. Mikrobiologiya 73:195–198
Krechel A, Faupel A, Johannes H, Ulrich A, Berg G (2002) Potato-associated bacteria and their antagonistic potential towards plant-parasitic fungi and the plant parasitic nematode Meloidogyne incognita (Kofoid & White) chitwood. Can J Microbiol 48:772–786
Liu XZ, Li SD (2004) Fungal secondary metabolites in biological control of crop pests. In: An ZQ (ed) Handbook of industrial mycology. Dekker, New York, pp 723–747
Liu B, Liu XZ, Zhuang WY (2005) Orbilia querci sp. nov. and its knob-forming nematophagous anamorph. FEMS Microbiol Lett 245:99–105
Manulis S, Shafrir H, Epstein E, Lichterl Barash I (1994) Biosynthesis of indole-3- acetic acid via the indole-3-acetarnide pathway in Streptomyces SPP. Microbiol 140:1045–1050
McGovern RJ, Mcsorley R, Bell ML (2002) Reduction of landscape pathogens in Florida by soil solarization. Plant Dis 86:1388–1395
Milagres AMF, Machuca A, Napaleao D (1999) Detection of siderophore production from several fungi and bacteria by a modification of chrome azurol S (CAS) agar plate assay. J Microbiol Methods 37:1–6
Mishra SK, Keller JE, Miller JR, Heisey RM, Nair MG, Putnam AR (1987) Insecticidal and nematicidal properties of microbial metabolites. J Ind Microbiol Biotechnol 2:267–276
Montealegre JR, Reyes JR, Perez LM, Herrera R, Silva P, Besoain X (2003) Selection of bio-antagonistic bacteria to be used in biological control of Rhizoctonia solani in tomato. Electron J Biotechnol 6:115–127
Nakahara S, Kusano M, Fujioka S, Shimada A (2004) Penipratynolene, a novel nematicide from Penicillium bilaiae chalabuda. Biosci Biotechnol Biochem 68:257–259
Noonim P, Mahakarnchanakul W, Varga J, Frisvad JC, Samson RA (2008) Two novel species of Aspergillus section Nigri from Thai coffee beans. Int J Syst Evol Microbiol 58:1727–1734
Ortiz A, Ordus Z (2001) In vitro evaluation of Trichoderma and Gliocladium antagonism against the symbiotic fungus of the leaf-cutting ant Atta cephalotes. Mycopathologia 150:53–60
Pandy A, Palni LMS (2007) The rhizosphere effect in trees of the India Central Himalaya with special reference to altitude. Appl Ecol Environ Res 5:93–102
Perez-Miranda S, Cabirol N, George-Tellez R, Zamudio-Rivera LS, Fernandez FJ (2007) O-CAS, a fast and universal method for siderophore detection. J Microbiol Methods 70:127–131
Roccuzzo G, Ciancio A, Bonsignore R (1993) Population density and soil antagonists of Meloidogyne hapla infecting kiwi in southern Italy. Fundam Appl Nematol 16:151–154
Samac DA, Kindel LL (2001) Suppression of the root-lesion nematode (Pratylenchus penetrans) in alfalfa (Medicago sativa) by Streptomyces spp. Plant Soil 235:35–44
Sembiring L, Ward AC, Goodfellow M (2000) Selective isolation and characterization of members of the Streptomyces violaceusniger clade associated with the roots of Paraserianthes falcataria. Antonie Van Leeuwenhoek 78:353–366
Sergeeva E, Liaimer A, Bergman B (2002) Evidence for production of the phytohormone indole-3-acetic acid by cyanobacteria. Planta 215:229–238
Sun MH, Gao L, Shi YX, Li BJ, Liu XZ (2006) Fungi and actinomycetes associated with Meloidogyne spp. eggs and females in China and their biocontrol potential. J Invertebr Pathol 93:22–28
Thakur MS, Vyas KM (1983) Production of plant growth regulators by some Fusarium species. Folia Microbiol (Praha) 28:124–129
Topp E, Miller S, Bork H, Welsh M (1998) Effects of marigold (Tagetes sp.) roots on soil microorganisms. Biol Fertil Soils 27:149–154
Tsavkelova EA, Klimova SY, Cherdyntseva TA, Netrusov AI (2006) Microbial producers of plant growth stimulators and their practical use: a review. Appl Biochem Microbiol 42:117–126
Wang LF, Yang BJ, Li CD (2001) Investigation of parasitic fungi on root-knot nematodes in East China. Mycosystema 20:264–267
Watanabe T (2001) Pictorial atlas of soil and seed fungi morphologies of cultured fungi and key to species, 2nd edn. CRC Press, New York
You JL, Cao LX, Liu GF, Zhou SN, Tan HM, Lin YC (2004) Isolation and characterization of actinomycetes antagonistic to pathogenic Vibrio spp. from nearshore marine sediments. World J Microbiol Biotechnol 21:679–682
Zhang KQ, Gowen S, Barbara T (1993) Root knot nematode-destroying fungi and the screening of effective strains. Acta Mycol Sin 12:240–245
Acknowledgments
This research was supported by The Royal Golden Jubilee Ph. D. Program (PHD/0064/2549), The Commission on Higher Education, Thailand on Target Research Initiative Program and Graduate School, Chiang Mai University. We are grateful to Professor John Peberdy, University of Nottingham and Arjan Morakot Sukchotiratana, Chiang Mai University for improving the English text.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruanpanun, P., Tangchitsomkid, N., Hyde, K.D. et al. Actinomycetes and fungi isolated from plant-parasitic nematode infested soils: screening of the effective biocontrol potential, indole-3-acetic acid and siderophore production. World J Microbiol Biotechnol 26, 1569–1578 (2010). https://doi.org/10.1007/s11274-010-0332-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-010-0332-8