Abstract
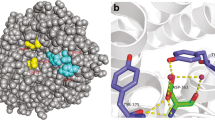
To investigate the roles of the active site residues in the catalysis of Bacillus thuringiensis WB7 chitinase, twelve mutants, F201L, F201Y, G203A, G203D, D205E, D205N, D207E, D207N, W208C, W208R, E209D and E209Q were constructed by site-directed mutagenesis. The results showed that the mutants F201L, G203D, D205N, D207E, D207N, W208C and E209D were devoid of activity, and the loss of the enzymatic activities for F201Y, G203A, D205E, W208R and E209Q were 72, 70, 48, 31 and 29%, respectively. The pH-activity profiles indicated that the optimum pH for the mutants as well as for the wildtype enzyme was 8.0. E209Q exhibited a broader active pH range while D205E, G203A and F201Y resulted in a narrower active pH range. The pH range of activity reduced 1 unit for D205E, and 2 units for G203A and F201Y. The temperature-activity profiles showed that the optimum temperature for other mutants as well as wildtype enzyme was 60°C, but 50°C for G203A, which suggested that G203A resulted in a reduction of thermostability. The study indicated that the six active site residues involving in mutagenesis played an important part in WB7 chitinase. In addition, the catalytic mechanisms of the six active site residues in WB7 chitinase were discussed.






Similar content being viewed by others
References
Aronson NN, Blanchard CJ, Madura JD (1997) Homology modeling of glycosyl hydrolase family 18 enzymes and proteins. J Chem Inf Comput Sci 37:999–1005
Aronson NN Jr, Halloran BA, Alexeyev MF, Zhou XE, Wang Y, Meehan EJ, Chen L (2006) Mutation of a conserved tryptophan in the chitin-binding cleft of Serratia marcescens chitinase A enhances transglycosylation. Biosci Biotechnol Biochem 70:243–251
Bhattacharya D, Nagpure A, Gupta RK (2007) Bacterial chitinases: properties and potential. Crit Rev Biotechnol 27:21–28
Bravo A (1997) Phylogenetic relationships of Bacillus thuringiensis delta-endotoxin family proteins and their functional domains. J Bacteriol 179:2793–2801
Bravo A, Gill SS, Soberón M (2007) Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 49:423–435
Candas M, Loseva O, Oppert B, Kosaraju P, Bulla LA Jr (2003) Insect resistance to Bacillus thuringiensis alterations in the indianmeal moth larval gut proteome. Mol Cell Proteomics 2:19–28
Chen J, Hua G, Jurat-Fuentes JL, Abdullah MA, Adang MJ (2007) Synergism of Bacillus thuringiensis toxins by a fragment of a toxin-binding cadherin. Proc Natl Acad Sci U S A 104:13901–13906
Downing KJ, Leslie G, Thomson JA (2000) Biocontrol of the sugarcane borer Eldana saccharina by expression of the Bacillus thuringiensis cry1Ac7 and Serratia marcescens chiA genes in sugarcane-associated bacteria. Appl Environ Microbiol 66:2804–2810
Fernández LE, Gómez I, Pacheco S, Arenas I, Gilla SS, Bravo A, Soberón M (2008) Employing phage display to study the mode of action of Bacillus thuringiensis Cry toxins. Peptides 29:324–329
Ferre J, Van Rie J (2002) Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu Rev Entomol 47:501–533
Grossi-de-Sa MF, Quezado de Magalhaes M, Silva MS, Silva SM, Dias SC, Nakasu EY, Brunetta PS, Oliveira GR, Neto OB, Sampaio de Oliveira R, Soares LH, Ayub MA, Siqueira HA, Figueira EL (2007) Susceptibility of Anthonomus grandis (cotton boll weevil) and Spodoptera frugiperda (fall armyworm) to a cry1Ia-type toxin from a Brazilian Bacillus thuringiensis strain. J Biochem Mol Biol 40:773–782
Hashimoto M, Honda Y, Nikaidou N, Fukamizo T, Watanabe T (2000a) Site-directed mutagenesis of Asp280 suggests substrate-assisted catalysis of chitinase A1 from Bacillus circulans WL-12. J Biosci Bioeng 89:100–102
Hashimoto M, Ikegami T, Seino S, Ohuchi N, Fukada H, Sugiyama J, Shirakawa M, Watanabe T (2000b) Expression and characterization of the chitin-binding domain of chitinase A1 from Bacillus circulans WL-12. J Bacteriol 182:3045–3054
Henrissat B, Bairoch A (1993) New families in the classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J 293:781–788
Hofte H, Whiteley HR (1989) Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev 53:242–255
Janmaat AF, Myers J (2003) Rapid evolution and the cost of resistance to Bacillus thuringiensis in greenhouse populations of cabbage loopers, Trichoplusia ni. Proc Biol Sci 270:2263–2270
Janmaat AF, Wang P, Kain W, Zhao JZ, Myers J (2004) Inheritance of resistance to Bacillus thuringiensis subsp. kurstaki in Trichoplusia ni. Appl Environ Microbiol 70:5859–5867
Kramer KJ, Muthukrishnan S (1997) Insect chitinases: molecular biology and potential use as biopesticides. Insect Biochem Mol Biol 27:887–900
Lin FP, Chen HC, Lin CS (1999) Site-directed mutagenesis of Asp313, Glu315, and Asp391 residues in chitinase of Aeromonas caviae. IUBMB Life 48:199–204
Lonhienne T, Mavromatis K, Vorgias CE, Buchon L, Gerday C, Bouriotis V (2001) Cloning, sequences, and characterization of two chitinase genes from the Antarctic Arthrobacter sp. strain TAD20: isolation and partial characterization of the enzymes. J Bacteriol 183:1773–1779
Lu Y, Zen KC, Muthukrishnan S, Kramer KJ (2002) Site-directed mutagenesis and functional analysis of active site acidic amino acid residues D142, D144 and E146 in Manduca sexta (tobacco hornworm) chitinase. Insect Biochem Mol Biol 32:1369–1382
Merzendorfer H, Zimoch L (2003) Chitin metabolism in insects: structure, function and regulation of chitin synthases and chitinases. J Exp Biol 206:4393–4412
Orikoshi H, Baba N, Nakayama S, Kashu H, Miyamoto K, Yasuda M, Inamori Y, Tsujibo H (2003) Molecular analysis of the gene encoding a novel cold-adapted chitinase (ChiB) from a marine bacterium, Alteromonas sp. strain O-7. J Bacteriol 185:1153–1160
Perrakis A, Tews I, Dauter Z, Oppenheim AB, Chet I, Wilson KS, Vorgias CE (1994) Crystal structure of a bacterial chitinase at 2.3 Å resolution. Structure 2:1169–1180
Reyes-Ramírez A, Ibarra JE (2008) Plasmid patterns of Bacillus thuringiensis type strains. Appl Environ Microbiol 74:125–129
Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler DR, Dean DH (1998) Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev 62:775–806
Smirnoff WA (1971) Effect of chitinase on the action of Bacillus thuringiensis. Can Entomol 103:1829–1831
Souza RF, Gomes RC, Coelho RR, Alviano CS, Soares RM (2003) Purification and characterization of an endochitinase produced by Colletotrichum gloeosporioides. FEMS Microbiol Lett 222:45–50
Takuo M, Takamasa N, Masayuki H, Takeshi W, Yukio M (1999) Three-dimensional structure of the catalytic domain of chitinase Al from Bacillus circulars WL-12 at a very high resolution. Proc Jpn Acad 75:269–274
Tantimavanich S, Pantuwatana S, Bhumiratana A, Panbangred W (1997) Cloning of a chitinase gene into Bacillus thuringiensis subsp. aizawai for enhanced insecticidal activity. J Gen Appl Microbiol 43:341–347
Terwisscha van Scheltinga AC, Hennig M, Dijkstra BW (1996) The 1.8 Å resolution structure of hevamine, a plant chitinase/lysozyme, and analysis of the conserved sequence and structure motifs of glycosyl hydrolase family 18. J Mol Biol 262:243–257
Thomas CJ, Gooday GW, King LA, Possee RD (2000) Mutagenesis of the active site coding region of the Autographa californica nucleopolyhedrovirus chiA gene. J Gen Virol 81:1403–1411
Watanabe T, Kobori K, Miyashita K, Fujii T, Sakai H, Uchida M, Tanaka H (1993) Identification of glutamic acid 204 and aspartic acid 200 in chitinase A1 of Bacillus circulans WL-12 as essential residues for chitinase activity. J Biol Chem 268:18567–18572
Watanabe T, Uchida M, Kobori K, Tanaka H (1994) Site-directed mutagenesis of the Asp-197 and Asp-202 residues in chitinase A1 of Bacillus circulans WL-12. Biosci Biotechnol Biochem 58:2283–2285
Wiwat C, Thaithanun S, Pantuwatana S, Bhumiratana A (2000) Toxicity of chitinase- producing Bacillus thuringiensis ssp. kurstaki HD-1 (G) toward Plutella xylostella. J Invertebr Pathol 76:270–277
Yang Y, Chen H, Wu Y, Yang Y, Wu S (2007) Mutated cadherin alleles from a field population of Helicoverpa armigera confer resistance to Bacillus thuringiensis toxin Cry1Ac. Appl Environ Microbiol 73:6939–6944
Yu X, Huang T, Huang Z, Powell CA, Guan X (2007) Expression and characterization of inhA gene from Bacillus thuringiensis 8010. World J Microbiol Biotechnol 23:1621–1625
Zhang H, Huang X, Fukamizo T, Muthukrishnan S, Kramer KJ (2002) Site-directed mutagenesis and functional analysis of an active site tryptophan of insect chitinase. Insect Biochem Mol Biol 32:1477–1488
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (30571257) and the Natural Science Foundation of Fujian Province (2007J0324).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, W., Sha, L., Zhou, J. et al. Functional analysis of active site residues of Bacillus thuringiensis WB7 chitinase by site-directed mutagenesis. World J Microbiol Biotechnol 25, 2147–2155 (2009). https://doi.org/10.1007/s11274-009-0119-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-009-0119-y