Abstract
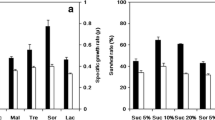
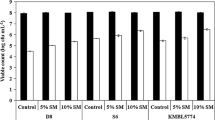
Malolactic fermentation (MLF) is an important process in wine production. To achieve successful MLF, expanding interest in ready-to-use Oenococcus oeni starter cultures has placed greater emphasis on developing starter production and preservation methods. In this study, influences of protectants, rehydration media and storage on the viability of O. oeni H-5 when subjected to freeze-drying were investigated. It was found that sodium glutamate (2.5%) was the best protectant, giving the cell viability 72.4%. Adding polysaccharides and disaccharides in suspension media also improved significantly the cell viability. Rehydration is an important step in recovery after freeze-drying. When freeze-dried O. oeni was rehydrated in GYM medium, the highest viability (87.1%) was obtained. Rehydration in the disaccharide solutions tested made the cell viability obviously decrease. After 6 months storage at 4°C, loss of viability occurred, the extent of which depended on protectants used, sodium glutamate again being the most effective.
Similar content being viewed by others
References
Baumann DP, Reinbold GW (1964) Preservation of lactic cultures. J Dairy Sci 47:674–676
Bozoglu TF, Ozilgen M, Bakir U (1987) Survival kinetics of lactic acid starter cultures during and after freeze-drying. Enzyme Microb Technol 9:531–537
Burke MJ (1986) The glassy state and survival of anhydrous biological systems. In: Leopold AC (ed) Membranes, metabolism and dry organisms. Cornell University Press, Ithaca, pp 358–363
Carpenter JF, Crowe JH, Arakawa T (1990) Comparison of the solute-induced protein stabilization in aqueous solution and in frozen and dried states. J Dairy Sci 73:3627–3636
Carvalho AS, Silva J, Ho P, Teixeira P, Malcata X, Gibbs P (2003) Effect of various growth media upon survival during storage of freeze-dried Enterococcus faecalis and Enterococcus durans. J Appl Microbiol 94:947–952
Champagne CP, Gardner N, Brochu E, Beaulieu Y (1991) The freeze-drying of lactic acid bacteria: a review. Can Inst Sci Technol J 24:118–128
Champagne CP, Mondou F, Raymond Y, Roy D (1996) Effect of polymers and storage temperature on the stability of freeze-dried lactic acid bacteria. Food Res Int 29:555–562
Costa E, Usall J, Teixido N, Garcia N, Vinas I (2000) Effect of protective agents, rehydration media and initial cell concentration on viability of Pantoea agglomerans strain CPA-2 subjected to freezedrying. J Appl Microbiol 89:793–800
Crowe JH, Crowe LM (1986) Stabilization of membranes in anhydrobiotic organisms. In: Leopold AC (ed) Membranes, metabolism and dry organisms. Cornell University Press, Ithaca, pp 188–209
Davis CR, Wibowo D, Eschenbruch R, Lee TH, Fleet GH (1985) Practical implications of malolactic fermentation: a review. Am J Enol Vitic 36:290–301
Font de Valdez G, de Giori GS, de Ruiz Holgado AP, Oliver G (1983a) Comparative study of the efficiency of some additives in protecting lactic acid bacteria against freeze-drying. Cryobiology 20:560–566
Font de Valdez G, de Giori GS, de Ruiz Holgado AP, Oliver G (1983b) Protective effect of adonitol on lactic acid bacteria subjected to freeze drying. Appl Environ Microbiol 45:302–304
Font de Valdez G, de Girori GS, de Ruiz Holgado AP, Oliver G (1985a) Rehydration conditions and viability of freeze-dried lactic acid bacteria. Cryobiology 22:574–577
Font de Valdez G, de Giori GS, de Ruiz Holgado AP, Oliver G (1985b) Effect of the rehydration medium on the recovery of freeze-dried lactic acid bacteria. Appl Environ Microbiol 50:1339–1341
Hubálek Z (2003) Protectants used in the cryopreservation of microorganisms. Cryobiology 46:205–229
Izutsu K, Yoshioka S, Terao T (1993) Decreased proteinstabilizing effects of cryoprotectants due to crystallization. Pharm Res 10:1232–1237
Kim AI, Akers MJ, Nail SL (1998) The physical state of mannitol after freeze-drying: effects of mannitol concentration, freezing rate, and anoncrystallizing cosolute. J Pharm Sci 87:931–935
Leslie SB, Israeli E, Lighthart B, Crowe JH, Crowe LM (1995) Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl Environ Microbiol 61:3592–3597
Maicas S, Gil JV, Pardo I, Ferrer S (1999) Improvement of volatile composition of wines by controlled addition of malolactic bacteria. Food Res Int 32:491–496
Moriche T (1970) Nature and action of protective solutes in freeze drying of bacteria. In: Waston BD, Grossmann MM, Sandine GC (eds) Proceedings of the first international conference on culture collections. University Park Press, Tokyo, pp 121–125
Nielsen JC, Prahl C, Lonvaud-Funel A (1996) Malolactic fermentation in wine by direct inoculation with freeze-dried Leuconostoc oenos cultures. Am J Enol Vitic 47:42–48
Palmfeldt J, Hahn-Hägerdal B (2000) Influence of culture pH on survival of Lactobacillus reuteri subjected to freeze-drying. Int J Food Microbiol 55:235–238
Panoff JM, Thammavongs B, Guéguen M, Boutibonnes P (1998) Cold stress responses in mesophilic bacteria. Cryobiology 36:75–83
Ray B, Jezeski JJ, Busta FF (1971) Effect of rehydration on recovery, repair and growth of injured freeze-dried Salmonella anatum. Appl Microbiol 22:184–189
Record BR, Taylor R, Miller DS (1962) The survival of Escherichia coli on drying and rehydration. J Gen Microbiol 28:585–589
Sanders JM, Venema J, Kok J (1999) Environmental stress responses in Lactococcus lactis. FEMS Microbiol Rev 23:483–501
Sinha RN, Shukla AK, Madan L, Ranganathan B (1982) Rehydration of freeze-dried cultures of lactic streptococci. J Food Sci 47:668–669
Theunissen JJH, Stolz E, Michel MF (1993) The effects of medium and rate of freezing on the survival of chlamydias after lyophilization. J Appl Bacteriol 75:473–477
Wolfe J, Bryant G (1999) Freezing, drying, and/or vitrification of membrane-solute-water system. Cryobiology 39:103–129
Acknowledgments
The authors are grateful to The China Scholarship Council (CSC) for its financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, G., Zhang, G. Influences of protectants, rehydration media and storage on the viability of freeze-dried Oenococcus oeni for malolactic fermentation. World J Microbiol Biotechnol 25, 1801–1806 (2009). https://doi.org/10.1007/s11274-009-0080-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-009-0080-9