Abstract
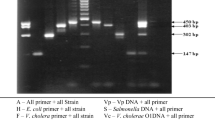
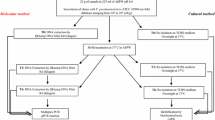
A rapid and sensitive 8-h PCR assay has been developed for detection of Salmonella serovars in seafood. A total of 110 fresh and raw seafood samples were analysed for the presence of Salmonella using different enrichment periods prior to PCR assay. Seafood samples included in this study were fish, shrimps, mussels, crabs, edible oysters, and clams, collected from local fish markets in Cochin (India). The assay was performed with a Salmonella-specific 284 bp invA gene amplicon. Specificity and sensitivity of the assay were ascertained with seafoods spiked with viable Salmonella cells to a level of 106 to 2 CFU per 25 g. Detection efficiency of the assay increased with increasing enrichment period for seafood, and 33.6% of seafood samples were found positive for Salmonella by 8-h PCR assay. Detection limit for the 8-h PCR assay showed visible 284 bp amplicon from seafood homogenates spiked with 2 CFU per 25 g. Seafood samples spiked with different Salmonella serovars, namely Salmonella typhi, Salmonella typhimurium, Salmonella enteritidis, Salmonella mbandka, Salmonella bareilly, and Salmonella weltevreden, were detected, confirming this technique would be ideal for detection of the Salmonella serovars prevalent in seafood. This study also covered inhibition by the seafood matrix and the detection limit for dead Salmonella cells during the PCR assay. There was no visible inhibition of this Salmonella PCR assay by seafood matrices. The detection limit for dead Salmonella cells by 8-h PCR assay was 2 × 103 CFU per 25 g seafood. The data indicated that dead cells of Salmonella in naturally contaminated seafood samples do not interfere with the assay resulting in false positives.


Similar content being viewed by others
References
Andrews WH, Hammack TS (1998) Salmonella, chapter 5, In Bacteriological analytical manual, U.S. Food and Drug Administration, 8th Edition, Revision A, AOAC International, Gaithersburg, Md
Chiu TH, Chen TR, Hwang WZ, Tsen HY (2005) Sequencing of an internal transcribed spacer region of 16S-23S rRNA gene and designing of PCR primers for the detection of Salmonella spp. in food. Int J Food Microbiol 97:259–265
Fach P, Dilasser F, Grout J, Tache J (1999) Evaluation of a polymerase chain reaction –based test for detecting Salmonella spp. in food samples: Probelia Salmonella spp. J Food Protect 62:1387–1393
Ferretti R, Mannazzu I, Cocolin L, Comi G, Clementi F (2001) Twelve-hour based method for detection of Salmonella spp. in food. Appl Environ Microbiol 67:977–978
Hatta AAM, Lakshmanaperumalsamy P (1997) Prevalence of Salmonella in fish and crustaceans from markets in Coimbatore, South India. Food Microbiol 14:111–114
Heinitz ML, Ruble RD, Wagner DE, Tatini SR (2000) Incidence of Salmonella in fish and seafood. J Food Protect 63:579–592
Iyer TSG, Shrivastava KP (1989) On the pattern of Salmonella serotypes in fishery products, froglegs and processing environments. Fish Technol 26:131–137
Kumar HS, Sunil R, Venugopal MN, Karunasagar I, Karunasagar I (2003) Detection of Salmonella spp. in tropical seafood by polymerase chain reaction. Int J Food Microbiol 88:91–95
Makino S, Kurazono H, Chongsanguam M, Hayashi H, Cheun H, Suzuki S, Shirahata T (1999) Establishment of the PCR system specific to Salmonella spp. and its application for the inspection of food and fecal samples. J Vet Med Sci 61:1245–1247
Martinez-Urtaza J, Saco M, Hernandez-Cordova G, Lonazo A, Garcia Martin O, Espinosa J (2003) Identification of Salmonella serovars isolated from live molluscan shellfish and their significance in the marine environment. J Food Protect 66:226–232
Malorny B, Hoorfar J, Bunge C, Helmuth R (2003) Multicenter validation of the analytical accuracy of Salmonella PCR, towards an international standard. Appl Environ Microbiol 69:290–296
Nambiar VN, Iyer KM (1991) Distribution of Salmonella serotypes in fish in retail trade in Cochin. Fish Technol 28:33–37
Rahn K, De Grandis SA, Clarke RC, McEwen SA, Galan JE, Ginocchio C, Curtis III R, Gyles CL (1992) Amplification of an invA gene sequence of Salmonella Typhimurium by polymerase chain reaction as a specific method of detection of Salmonella. Mol Cell Probe 6:271–279
Rattagool P, Wongchinda N, Sanghtong N (1990) Salmonella contamination in Thai shrimp. FAO Fishery Report vol 401. Supplement, FAO, Rome. pp 18–23
Saheki K, Kabayshi S, Habanishi T (1989) Salmonella contamination of eel culture ponds. Nippon Suisan Gakkai Shi 55:675–679
Tietjen M, Fung DYC (1995) Salmonellae and food safety. Critic Rev Microbiol 21:53–83
Yam WC, Chan CY, Ho Bella SW, Tam TY, Kueh C, Lee T (1999) Abundance of clinical enteric bacterial pathogens in coastal waters and shellfish. Water Res 56:51–56
Acknowledgements
Authors are thankful to the Director, CIFT, for necessary facilities and kind permission to publish this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, R., Surendran, P.K. & Thampuran, N. An eight-hour PCR-based technique for detection of Salmonella serovars in seafood. World J Microbiol Biotechnol 24, 627–631 (2008). https://doi.org/10.1007/s11274-007-9513-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-007-9513-5