Abstract
Invasive plants are a threat to natural ecosystems worldwide, with urban wetlands being some of the most susceptible and highly modified environments of all. The tiger snake (Notechis scutatus) is a top predator that persists in urban wetlands of south-western Australia, many of which have been degraded by introduced kikuyu grass (Cenchrus clandestinus). To evaluate the potential impact of kikuyu grass on habitat quality for tiger snakes we quantified the structural features of habitats within wetlands degraded by kikuyu grass and compared them to wetlands with native vegetation. We also examined tiger snake prey availability, assessed predation risk for juvenile snakes using clay models, and measured the thermal quality of the vegetation. Proliferation of kikuyu grass has reduced habitat structural heterogeneity by reducing available bare ground and increasing vegetation density. This homogenisation of habitat structure had little effect on the predation risk for juveniles or the thermal properties of tiger snake shelter sites; however, one key prey species, the motorbike frog, had significantly lower abundance in the most impacted habitat. Habitat types with more structural complexity also offered tiger snakes more stable thermal regimes and lower predation risk. These findings indicate that the current extent of kikuyu grass invasion offers overall similar habitat quality for tiger snakes to native vegetation and may contribute to their persistence in urban wetlands; however, both tiger snakes and their anuran prey may benefit from increased habitat structural complexity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urbanisation is a major driver of global environmental change, with urban infrastructure and human populations encroaching on and modifying natural ecosystems (Faulkner 2004; Coleman 2017; Cresswell and Murphy 2017). Urbanisation leads to the degradation and removal of natural spaces for plants and animals and the introduction of invasive of species which create new biological interactions and alter the physical environment (McDonnell et al. 1993; Kinzig and Grove 2001; Hamer and McDonnell 2008; Štajerová et al. 2017). Wetlands are an ecosystem type particularly threatened by urbanisation. Wetland ecosystems are the most rapidly declining ecosystems world-wide (Balmford et al. 2002; Sieben et al. 2016) and even international treaties such as the Ramsar Convention and identification of World Heritage areas, that recognise their importance for the conservation of biodiversity, have failed to halt this decline, which is anticipated to accelerate further with the additional impacts of climate change (Nicholls et al. 1999; Burgin et al. 2016). In Australia, natural wetlands have been lost from much of the continent, with areas of particular concern including the south-eastern and south-western regions of the continent, where increasing urbanisation means wetlands are lost or highly modified to accommodate concentrated anthropogenic land uses (Burgin et al. 2016). On the Swan Coastal plain in south-west Western Australia, 70% of wetlands have been lost by activities that facilitate urban development, including drainage, clearing of native vegetation and road construction (Lemly et al. 2000).
Management and protection of remaining wetlands is essential for conservation in urban areas because of their importance to the maintenance of urban biodiversity (Burgin et al. 2016). Wetland habitats are especially vulnerable to invasive plants as up to a quarter of the most invasive plant species worldwide are wetland species, which then benefit from accumulation of materials, such as sediment and nutrients, in wetland habitats (Zedler and Kercher 2004). Invasive plants are a threat to ecosystems globally and can dominate disturbed or sensitive vegetation communities, reducing plant diversity and structural heterogeneity and impacting ecosystem function (Schweiger et al. 2018) and retention of biodiversity (Stein et al. 2014). Invasive wetland weeds are particularly prone to forming monocultures (Zedler and Kercher 2004) and these monocultures can reduce habitat quality for fauna by reducing structural complexity and resource availability, impacting predation pressure, thermal quality, food availability and ultimately the overall suitability of the habitat (Huey 1991; Webb et al. 2005; Lee et al. 2006). Invasion of wetlands by weeds typically reduces mammal, bird, fish and insect diversity and abundance, and in particular impacts on species that are habitat specialists (Zedler and Kercher 2004).
Large reptiles are the top predators of many Australian ecosystems, with diverse effects on ecosystem structure and function (Pianka 1986; Kuch et al. 2005; Read and Scoleri 2015; Doody et al. 2021). Tiger snakes (Notechis scutatus) are large, venomous elapid snakes of southern Australia that are typically affiliated with wetland habitats. These wetlands support populations of frogs (the primary prey of tiger snakes) and waterbodies that are used by tiger snakes to evade predators (Aubret 2004; Lettoof et al. 2020). Tiger snakes can be a conspicuous top predator in anthropogenic landscapes where their presence and abundance is sensitive to an intact food web and habitat availability (Sergio et al. 2008); thus they are considered bioindicators of wetland health (Lettoof et al. 2022). Western tiger snakes (N. s. occidentalis) persist in a subset of urban wetlands within the urban sprawl of Perth city (Lettoof et al. 2022).
Some of the urban wetlands inhabited by western tiger snakes are dominated by introduced kikuyu grass (Cenchrus clandestinus), a globally recognised invasive species, particularly in wetlands (Bird et al. 2013; Boon and Tesfamichael 2017). The aggressively competitive nature of many introduced grass species and their tolerance of above-ground disturbance and sedimentation makes them particularly successful invasive species (Zedler and Kercher 2004; Ferdinands et al. 2005; Sieben et al. 2016). Abundant kikuyu lowers native plant species richness and cover (Gaertner et al. 2011) and has potentially similar impacts on fauna. For example, in wetlands in northern Australia, birds avoid areas dominated by invasive para grass (Urochloa mutica) in favour of more heterogenous natural habitat (Ferdinands et al. 2005). However, not all fauna is negatively affected by the invasion of exotic grasses (Douglas et al. 2006). For some reptiles, habitat structure is more important than plant species composition (Garden et al. 2007) and populations may persist in highly modified habitats if structural components are retained within the landscape. For example, the presence of structurally complex habitats created by the invasive woody shrub lantana (Lantana camara) supports large numbers of a rare species of shadeskink (Saproscincus rosei; Virkki et al. 2012). Invasive grasses such as kikuyu may also provide suitable habitat for the frogs on which tiger snakes prey (Zavaleta et al. 2001; Maerz et al. 2005) and prey availability is an important driver of snake abundance and habitat use (McCauley et al. 2006; Battles et al. 2013; Zipkin et al. 2020).
A broadly inhospitable matrix of urban area restricts tiger snakes on the Swan Coastal Plain to the wetlands they inhabit (Lettoof et al. 2021b), with little opportunity for individuals to move between habitat patches. Consequently, the quality of these wetlands and availability of prey are factors crucial for maintenance of viable populations of this top predator. An over-abundance of kikuyu appears to have substantially changed the vegetation composition and habitat structure at many of these wetlands. The flow-on effects for prey abundance, predation risk and thermal quality for tiger snakes has not been determined so here we investigate the impacts of kikuyu invasion on these variables for wetlands, by comparing a range of biotic and abiotic metrics in kikuyu-dominated wetlands against wetlands that are dominated by native vegetation communities. We quantify vegetation structure, prey availability (frog abundance), predation risk (for juveniles) and thermal quality of available microhabitats to better understand how kikuyu might impact wetland fauna and assist land managers to better manage degraded urban wetlands to maximise wetland ecosystem function and faunal biodiversity.
Methods
Study sites
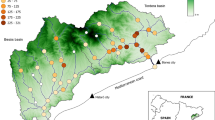
We examined western tiger snake habitats at two wetlands dominated by kikuyu and two wetlands dominated by native vegetation, all within 50 km of the Perth CBD, in south-west Western Australia (Fig. 1). Western tiger snake populations have been previously documented as occurring at these wetlands (Lettoof et al. 2021a). At Herdsman Lake (HL; 31.92°S, 115.80°E) and Kogolup Lake (KL; 32.12°S, 115.83°E) kikuyu was the dominant ground cover vegetation while at Loch McNess in Yanchep National Park (Y; 31.54°S, 115.68°E) and Black Swan Lake (BS; 32.47°S, 115.77°E) native vegetation predominated. Although the total area of the wetlands varied greatly the area of potential tiger snake habitat at these wetlands was similar for each site, ranging from 49.1 ha (HL) to 59 ha (Y; Cornelis et al. 2023). We were limited to four wetland sites due to the requirement for sites that (1) had a distinctly kikuyu or native species dominated vegetation, (2) a substantial tiger snake population, (3) were accessible for our study and (4) occurred on the Swan Coastal Plain within the urban sprawl of the Perth metropolitan area. Within these four wetland sites, we identified a priori three broad riparian habitat categories occurring within 50 m of the waterbody; open grassland (abundant ground cover of either uncut kikuyu grass or native grasses with no canopy cover or mid-story vegetation), sedge (mid-story level dense native sedges or uncut kikuyu grass growing against bullrush bordering waterbodies), and woodland habitats (canopy cover and understory vegetation, occasionally with mid-story vegetation; Fig. S1) and use the terms ‘kikuyu’ or ‘native’ to distinguish the broad wetland communities (i.e. two kikuyu dominated wetlands and two native-vegetation dominated wetlands) from the finer-scale riparian habitat categories (open, sedge and woodland) within these sites.
Wetlands that were surveyed in the Swan Coastal Plain. BS black swan lake, HL herdsman lake, KL kogolup lake, Y yanchep national park. Red font indicates wetlands classed as dominated by native vegetation, blue font indicates wetlands dominated by kikuyu grass. Satellite images were obtained from Google Earth in 2022. Scale bar = 500 m
Habitat structure
To quantify habitat structure, we recorded vegetation features along two 100 m transects in each habitat type at each wetland. Every 10 m along the transect we recorded canopy cover using a spherical densiometer (Forest Densiometers, Model A), light availability with a digital lux meter (DR.meter LX101BS, when the reading in ambient unshaded light was ca. 720,000 lx), the number of trees with stems > 150 mm circumference at breast height (1.4 m) within a 2 m radius, the number of woody plants with stems < 150 mm circumference at breast height within a 2 m radius, the height of the tallest understorey plant, an estimate of the percent ground cover within a 2 m radius and the vegetation density. Vegetation density was quantified by inserting a 50 cm × 50 cm board inside the vegetation (2 m from the observer), taking a photograph, and then using ImageJ 1.x (Schneider et al. 2012) to calculate the surface area of the board not obscured by vegetation. If the vegetation was too dense to insert the board we recorded vegetation density as 100% (Fox et al. 1996; Colman et al. 2014, 2015).
All statistical analyses were conducted in R studio version 1.4.1103 (RStudio Team 2020). We used principal component analysis (PCA) to examine associations between the three a priori riparian habitat types (open, sedge and woodland) and vegetation communities (kikuyu or native vegetation) based on the measured habitat characteristics. The FactoMineR (Lê et al. 2008) and factoextra (Kassambara and Mundt 2020) packages were used to generate principal components (PCs); PCs with eigenvalues > 1 were considered useful for inference (Roznik and Reichling 2021). An analysis of deviance was then used to compare the PCs of the riparian habitat types and to compare sites with kikuyu to those with native vegetation, with Tukey’s post-hoc pairwise comparisons (Roznik and Reichling 2021) achieved with the emmeans package (Lenth et al. 2018).
Prey availability
We measured the abundance of prey (frogs) in the different vegetation communities by conducting standardised 10 min active visual searches in the open, sedge and woodland riparian habitat types at the four wetlands. The search commenced in the centre of each habitat type and observers walked a steady pace while counting individual frogs detected with a spotlight. All transects were at least 100m apart, and surveyed once per night in a randomised order; Yanchep and Black Swan (native) were surveyed for five nights each, and Kogolup Lake and Herdsman Lake (kikuyu) were surveyed on four nights each, for a total of 54 visual searches. All transects were selected based on known presence of tiger snakes (JC & DL, pers. obvs.). Frogs were identified to species. Only a single wetland was surveyed each night to mitigate any temporal effects caused by time of night. Surveys were conducted in October and November (2019), which is peak calling season for the dominant prey species Litoria moorei, Litoria adelaidensis and Limnodynastes dorsalis (Hoskin et al. 2015; Lettoof et al. 2020). All surveys began after 19:00 on evenings when ambient temperature at this time was > 15 °C and were completed before 22:30.
Generalised linear mixed-effects models (GLMMs; lme4 package, Bates et al. 2014) with Tukey’s post-hoc pairwise comparisons were used to assess the differences in frog abundance among vegetation communities and habitats. We used a two-step process for assessing differences: Firstly, frog abundance was broadly compared between vegetation communities and habitat types; fitting a GLMM (Poisson error structure) with total frog count of the two most commonly observed species of frog present at all sites (slender tree frog and motorbike frog) per transect per night as the response variable and vegetation community (kikuyu or native vegetation) and habitat type (open, sedge or woodland) as fixed effects. Site and transect were included as random effects to account for the repeated surveys at each site on different days, after Crawley (2007). We also ran these models with just slender tree frog and just motorbike frog count as the response variable, respectively. The second approach was to compare the abundance of slender tree frog and motorbike frog with habitat structure variables (e.g. canopy and ground cover, light, tree density, understory cover etc.) to determine which specific structural components of a habitat type were influencing frog abundance. We fitted a GLMM (Poisson error structure) with frog count data as the response variable, the habitat structure variables as predictor variables, and site as a random effect. Predictor variables were first scaled and centred to improve model fitting and correlated variables were removed to reduce multicollinearity, retaining variables with variance inflation factors < 5 and pairwise correlations < 0.7, identified using the usdm and psych packages (Naimi 2015; Revelle 2015; Lettoof et al. 2020). For correlated variables the least ecologically relevant predictors were removed, after Dormann et al. (2013). The global model included canopy cover, vegetation density, vegetation height and woody vegetation as predictor variables. Model fit was then assessed with pseudo-R2 values calculated with the glmmADMB package (Skaug et al. 2013). Following this, model selection was performed using the dredge function from the MuMIn package (Barton 2015) and all sub models were ranked according to Akaike’s information criterion, with a sample size correction (AICc). All models with ΔAICc < 2 were considered useful for inference.
Predation risk
We assessed the differences in predation risk for young tiger snakes among vegetation communities and habitats using clay models. We moulded 120 soft clay models, representing juvenile tiger snakes because snakes are most vulnerable to predation when they are small (Lima and Dill 1990; Webb et al. 2005), and 120 spherical control balls from nontoxic Plastiplay™ brown modelling clay (Fig. S2A). Total body length of the artificial snakes was ca. 25 cm, width 1 cm and they were moulded into a sinusoidal shape. Control balls were 4 cm in diameter and used to determine if predators discriminated foreign objects from artificial snakes (Nordberg and Schwarzkopf 2019). The artificial snakes and balls were deployed at the four study sites in each of the three different habitat types. In each habitat type, ten artificial snakes were placed in exposed areas to simulate basking sites, and ten were hidden under vegetation to simulate refuge sites. Artificial snakes were separated by 5 m, with each model accompanied by a control ball at a distance of 25 cm. All clay models were checked every day for a 5 day period and marks left by predators were counted, scored by intensity (depth) of attack and the predators were identified as birds, rodents or cats, based on the shape of the marks left by the predators (Webb and Whiting 2005) or classified as unknown if the clay models were missing. Small shallow marks, often left by house mice (Mus musculus), were scored as exploratory rather than predatory as they did not represent a realistic predatory attempt on a juvenile tiger snake; these were therefore omitted from analyses (Fig. S2B). Experiments were conducted in autumn and repeated in spring for a total of 10 days of sampling at each site.
We used a Student’s t-test to compare the difference in total predation rate between snake models and control balls. We used the same two-step process used to analyse frog abundance data for assessing differences in predation counts both among vegetation communities and habitat types and for habitat structure variables. The response variable for these GLMMs was whether a model had experienced a predation attack (binomial yes/no). The global model included vegetation density, ground cover, light availability and vegetation height as predictor variables.
Thermal quality of vegetation
To determine if kikuyu grass or native vegetation differ in their thermal quality we monitored the temperatures within potential tiger snake shelter sites comprised of these either of these two types of vegetation with operative temperature models constructed using 30 cm long sections of copper pipe (32 mm diameter, wall 1 mm thick) filled with water and painted to approximate the reflectivity of a tiger snake (Seebacher and Shine 2004; Lutterschmidt and Reinert 2012). In the centre of each model we suspended a small temperature logger (Thermochron iButton) which recorded temperature at 10min intervals. The temperature logger was waterproofed (Plasti Dip) and wrapped in plastic film. Temperatures of the operative models were calibrated against tiger snake carcasses over a range of thermal and radiative conditions (Fig. S3). We deployed models in the field and calculated their thermal properties via a custom-written VB program (Visual Basic V6; Cooper and Withers 2004).
Three operative temperature models were deployed at each wetland, one in each of the three habitat types, placed within what we refer to as a shelter site (deep within the vegetation where we have observed tiger snakes retreating or emerging; pers obs.). Data were recorded at 10 min intervals for 7 days every month for 12 months. We calculated the mean, maximum and minimum operative environmental temperature (Te) of the sheltered microhabitats. Then we calculated the thermal quality (de) of tiger snake shelter sites as the absolute value of how much Te in shelter sites deviated from the thermal set-point range (Tset) for tiger snakes (Blouin‐Demers and Weatherhead 2002), determined by Ladyman and Bradshaw (2003) for snakes from Herdsman Lake in a thermal gradient as 24.0 ± 0.9 °C to 30.9 ± 0.3 °C. The resulting data were then grouped into four seasons and we compared the differences in thermal quality between sites with kikuyu grass and native vegetation and the habitat types within sites. To compare seasonal habitat temperature characteristics between vegetation types, we used a GLMM (Gaussian error structure) with the temperature characteristic as the response variable, vegetation type, habitat type and season as fixed predictor variables and site as a random effect to account for repeated measurements at each site. Tukey’s post-hoc pairwise comparisons were used to examine differences between variables.
Results
Habitat structure
Three PCs were retained (eigenvalue > 1) from the PCA analysis of habitat structure, collectively accounting for 73.7% of the total variance. PC1 explained 35.5% of the variance and was associated with canopy cover (Canopy), number of trees (Trees), woody vegetation (Woody) and low light availability (Lux; Table S1). PC2 explained 23.8% of the variance and was associated with ground cover (Ground), vegetation density (Density) and canopy cover. PC3 explained 14.4% of the variation and was associated with understory height. Collectively, the structure of kikuyu grass habitats was more homogenous than native vegetation habitats (Fig. 2) and differed significantly for PC2 (F5, 238 = 495, P < 0.001). The structure of our a priori riparian habitat types were significantly different for PC1 (F5, 234 ≤ 149, P < 0.001), although no pairwise differences were detected for kikuyu or native vegetation types (P ≥ 0.526). For PC3, open vegetation types were statistically similar (P = 0.856) for native and kikuyu dominated sites, but sedge and woodland riparian habitats differed for sites dominated by kikuyu compared to native vegetation (P < 0.001).
PCA ordination of habitat characteristics in western tiger snake (Notechis scutatus occidentalis) habitats from four different wetlands, dominated by either invasive vegetation (Herdsman Lake and Kogolop Lake) or native vegetation (Black Swan Lake and Yanchep National Park) in the Perth region, Western Australia. A The ordination of the habitat characteristics of the kikuyu grass and native vegetation plant communities, B ordination of the different habitat types within the wetlands KO kikuyu open, KS kikuyu sedge, KW kikuyu woodland, NO native open, NS native sedge, NW native woodland and. PC1 explains 35.5% and PC2 21% of the total variance. Arrows denote the influence of specified habitat characteristics and ellipses denote 80% of the spread of the groups
Prey availability
Two species of frog were frequently observed during surveys at all four study sites: slender tree frog (Litoria adelaidensis) and motorbike frog (Litoria moorei). Only two individuals of the rattling froglet (Crinia glauerti) were detected at a single site (Yanchep) and the moaning frog (Heleioporus eyrie) was not detected at Herdsman Lake (Table 1). Consequently, these two species were excluded from all subsequent analyses and we only investigated the abundance of slender tree frog and motorbike frog.
Total frog abundance (slender tree frog and motorbike frog combined) was not significantly influenced by vegetation community (kikuyu grass or native vegetation) or habitat type (open, sedge and woodland; χ2 2 ≤ 3.340, P ≥ 0.188); however, the interaction between vegetation community and habitat type did significantly influence total abundance of frogs (χ2 2 ≤ 26.55, P < 0.001), with a greater abundance of frogs in the native open habitat compared to the other native habitat types and the open kikuyu habitat; the open kikuyu habitat also had a significantly lower abundance of frogs than the kikuyu woodland habitat (post-hoc comparison P ≤ 0.003; Fig. 3). Further, there were significant differences in the abundance of frogs among habitat types within vegetation communities. Native open habitats had significantly more frogs than the native sedge and (NS) and native woodland habitats (NW; post-hoc comparisons P < 0.001) in sites dominated by native vegetation. In At sites dominated by kikuyu grass communities, total frog abundance was significantly lower in the open habitat compared to the kikuyu woodland (KW; post-hoc comparisons P = 0.001).
Model predicted mean (± SE) frog abundance in wetlands of Perth, Australia, dominated by either kikuyu grass or native vegetation. A Shows patterns in total frogs (all species summed), B shows patterns for slender tree frogs (Litoria adelaidensis) and C shows patterns for motorbike frogs (Litoria moorei). * denotes a significant difference between habitat types within vegetation communities
We observed larger numbers of slender tree frogs in native vegetation compared to kikuyu-dominated vegetation, although this was not significantly different (χ21 = 2.893, P = 0.088). However, habitat type and the interaction between vegetation community and habitat type significantly influenced abundance of the slender tree frog (χ2 2 ≤ 8.489, P < 0.040) with the native woodland having significantly lower abundance of slender tree frog than the native sedge and open habitat types (post-hoc comparison P ≤ 0.003). Motorbike frog abundance was not influenced by vegetation community or the interaction between vegetation community and habitat type (: χ2 2 ≤ 2.736, P ≥ 0.254); however habitat type did significantly influence motorbike frog abundance (χ22 = 7.940, P = 0.018) with woodland habitats having a greater abundance of motorbike frogs than open habitats (post-hoc comparison P ≤ 0.026). The abundance of both species was generally the same, for equivalent habitat types, for kikuyu and native vegetation dominated sites (post-hoc comparisons; P ≥ 0.072), except the slender tree frog was significantly more abundant in open habitats in sites dominated by native vegetation. Canopy cover, vegetation density and ground cover (%) were important predictors of both species; slender tree frog abundance was negatively associated with canopy cover and ground cover, and positively associated with vegetation density (Table 2), whereas motorbike frog abundance was positively associated with canopy cover, ground cover and understorey height and as was negatively associated with vegetation density (Table 2).
Predation risk
Of the 1200 observations of clay models, 57 predation attempts were recorded for artificial snakes and 17 for control balls. Artificial snakes were significantly more likely to be predated than control balls (t958 = 3.98, P < 0.001). Most predation marks could be identified as being a consequence of either birds (54%) or rodents (40%; Table 3). Due to the infrequency of predation attempts, we could not investigate differences in the frequency of different predator interactions with the models. All predation data were therefore pooled into a single response variable, ‘predation’.
There was no significant difference in the probability of predation on artificial snakes between vegetation communities (χ2 1 = 1.27, P = 0.257). The probability of predation was significantly different among habitat types (χ2 2 = 6.86, P = 0.032) although post-hoc comparisons of pairwise habitats showed no significant differences (P ≥ 0.270; Fig. 4). The structural variables of light availability and occurrence of woody vegetation were the best predictors of predation probability for artificial snakes, as these variables occurred in most of the five top models (Table 4). Predation probability increased with light availability and decreased with woody vegetation count.
Model predicted mean (± SE) probability of predation for clay model snakes in wetland vegetation communities dominated by either introduced kikuyu grass (Cenchrus clandestinus) or native vegetation in Perth, Australia. Predation attempts were quantified in three habitat types (open, sedge and woodland)
Thermal quality of vegetation
Calibration of the operative temperature models indicated they accurately represented the temperatures an actual tiger snake would attain (see description in Online Resource 1 and Fig. S3). Most of the thermal variables were highly correlated with demax (R ≥ 0.90), except for Te, max (R = 0.68) and de0 (proportion of time de was equal to 0; R = 0.43), so we only analysed vegetation and riparian habitat effects for these three variables. The Te,max differed seasonally (F3 = 413, P = < 0.001), being highest in summer and lowest in winter (Fig. 4). The Te,max in habitat types did not differ within each season (post-hoc comparisons P ≥ 0.190), except for at sites dominated by native vegetation, when open habitat had significantly higher Te,max than sedge habitat during summer (post-hoc comparisons P < 0.001; Fig. 5). The dominant vegetation type (kikuyu or native) had no influence on demax (F1 = 0.05, P = 0.837) but there was a significant difference in demax among habitat types (F2 = 31.8, P < 0.001) with the highest demaxs occurring in open habitats and the lowest demaxs in the woodland habitats (Fig. 5). Season also significantly influenced demax (F3 = 275.53, P < 0.001) which was lowest during summer, highest during winter and similar in autumn and spring (Fig. 5), with no interaction (F6 = 0.21, P = 0.972; Fig. 5). Neither the dominant vegetation type (F1 = 0.04, P = 0.857) or riparian habitat type (F2 = 1.69, P = 0.184) influenced the mean proportion of time that the thermal quality of the vegetation fell within Tset (de0). However there was a significant interaction between vegetation community, habitat type and season (F6 = 3.04, P = 0.005); within a season, there were no differences between equivalent habitat types in native or kikuyu vegetation (post-hoc comparisons P ≥ 0.240) but there were some differences between habitat types within the same vegetation community e.g. the highest mean de0 occurred in kikuyu-domianted woodland during summer and differed from open and sedge habitats in the same season (post-hoc comparisons P ≤ 0.001; Fig. 5).
Temperature parameters (A maximum operative environmental temperature; Te, B maximum deviation of environmental temperature, demax and C proportion of time the environmental temperature in sheltered microhabitats fell within the body temperature set-point range; de0) for tiger snakes (Notechis scutatus occidentalis) at four wetlands in Perth, Western Australia recorded during four seasons. Parameters were measured in sheltered microhabitats in three habitat types at sites where the vegetation is dominated by either introduced kikuyu grass or native vegetation
Discussion
We investigated a suite of habitat factors likely to influence populations of western tiger snakes in urban wetlands where native vegetation communities have been largely replaced by a single invasive species, kikuyu grass. Our results indicate that habitat compositional heterogeneity in wetlands with kikuyu grass has been reduced and that prey availability may be reduced in areas that have become dominated by kikuyu. However, despite the reduction in bare ground and increase in vegetation density at sites invaded by kikuyu, the transition from native vegetation to invasive grass appears to have had little effect on the predation risk for juvenile snakes or the thermal properties of shelter sites. Together, these findings indicate that the current extent of invasion of kikuyu grass in wetlands of the Swan Coastal Plain offers an overall similar habitat quality for tiger snakes to natural vegetation and is unlikely to substantially impact population persistence in these urban wetlands.
Habitat structure
Diverse habitat structure is a primary driver of biodiversity worldwide (Culbert et al. 2013; Boeye et al. 2014; von Takach et al. 2020). Our results indicated that habitat structure is similar between kikuyu-dominated and native-dominated vegetation, but is different between habitat types (open/sedge/woodland), supporting our a priori determination of habitat categories for the riparian vegetation within these wetlands. Increased vegetation density, ground cover and overall homogenisation drove the observed differences in the structure of kikuyu dominated vegetation communities compared to native vegetation equivalents. Kikuyu grows as a dense matrix of stems, which facilitates its colonisation and reduces inter-plant distance compared to native grasses, resulting in less bare ground and reduced plant diversity, transforming native vegetation communities into a monoculture (Gonzalez 2009; Litt and Steidl 2011; Lindsay and Cunningham 2012; Bradshaw et al. 2013; Abom et al. 2015).
The domination of an environment by a monoculture of a single invasive grass can have adverse effects on the ecosystem. For example, the invasion of gamba (Andropogon gayanus) and para (Urochloa mutica) grasses in northern Australia results in higher fuel loads and higher intensity fires than in uninvaded habitats, due to higher biomass and lower moisture content of the invasive species (Rossiter et al. 2003; Douglas and O’Connor 2004; von Takach et al. 2022). The most common impact of habitat structural homogeneity from invasive grasses, however, is the loss of native plant (Stephens et al. 2008), invertebrate (Douglas and O’Connor 2003) and vertebrate (Ferdinands et al. 2005; Cook and Grice 2013; Stanton et al. 2018) biodiversity—largely attributed to a loss of suitable habitat usually provided by structurally complex vegetation communities. Similar to these studies, we found that the invasion of kikuyu grass changed the habitat structure of riparian wetland vegetation. While we quantified the impact of this change on two common frog species, we encourage further research into how this invasion may affect other wetland biota in these urban wetlands.
Prey availability
The primary difference we found between kikuyu and native-dominated vegetation was the native open habitats had a greater abundance of slender tree frog and motorbike frog (combined), than the kikuyu open equivalent. In general, current levels of kikuyu in the wetlands we investigated made no difference to the total abundance of frogs, despite frogs being amongst the most sensitive vertebrate taxa to anthropogenic habitat modification (Nowakowski et al. 2017). However, the abundance of slender tree frog and motorbike frog among habitat types differed within and between wetlands dominated by kikuyu compared to native vegetation. Slender tree frog had higher abundance in the native open habitats, with the lowest abundance found in open kikuyu habitats, consistent with the species having a negative response to anthropogenic disturbance (Liu et al. 2021). The motorbike frog however, is one of the most tolerant of Australian frogs to habitat modification; indeed it often benefits from anthropogenically altered environments (Liu et al. 2021). It is therefore not surprising that motorbike frogs are tolerant of kikuyu grass invasion. Their negative association with vegetation density explains their highest abundance in the woodland habitats and is presumably a trait that enhances their overall success in highly human-modified environments. Compared to slender tree frogs, motorbike frogs also have a larger body size, broader geographical distribution and larger clutch size, all traits associated with tolerance of modified habitats (Nowakowski et al. 2017; Liu et al. 2021). As motorbike frogs contribute a substantial proportion of tiger snake diet in these wetlands (Lettoof et al. 2020), their presence may explain why tiger snakes have persisted in these wetlands. However, the reduction in abundance of smaller (34–47 mm) slender tree frogs likely reduces prey availability for young tiger snakes in kikuyu-dominated habitats and may be of concern for the recruitment of juvenile snakes into populations.
We observed the lowest abundance of all frogs in the open kikuyu habitat, although it is possible that the dense structure created by kikuyu grass made it difficult to detect all individuals (Heard et al. 2008; Vences et al. 2008). This habitat type is a monoculture of dense grass, and structurally very different from the open native vegetation equivalent which is comprised of several structurally diverse plant species and interspersed with bare ground. Plant structure and composition can have substantial impacts on amphibian ecology as vegetation influences species interactions including predation and foraging opportunities, reproduction, disease and abiotic conditions such as temperature (Burrow and Maerz 2022). Invasive grass monocultures usually result in a loss of abundance and diversity of native birds (Catling 2005; Skórka et al. 2010), reptiles (Valentine et al. 2007; Hacking et al. 2014), frogs (Grant and Samways. 2016; DeVore and Maerz 2014; Falaschi et al. 2020) and rodents (Sammon and Wilkins. 2005) globally. The abundance of tiger snakes two dominant prey species was highest in native open, native sedge and both woodland habitats, where the greater structural complexity and woody vegetation is likely favourable (Landsman and Bowman 2017), potentially reducing predation risk (DeVore and Maerz 2014; Norbury and van Overmeire 2019). As large expanses of riparian wetland vegetation in Perth can be kikuyu grass monocultures (Hill et al. 1996; Department of Environment and Conservation 2012), the persistence of these frogs and their tiger snake predators could be facilitated by the planting and maintenance of riparian grasses (Schoenoplectus spp.), sedges (Gahnia decomposita and/or Lepidosperma longitudinale) and native woodland trees (Banksia, Melaleuca and Eucalyptus spp) to supplement and supress the dense kikuyu vegetation (e.g. Burrow and Maerz 2022).
Predation risk
Predation attempts on juvenile snake clay models occurred at low frequency, with birds and rodents the predominant predators. The low frequency of predation attempts (4.75% of 1200) on artificial juvenile tiger snakes is comparable to the predation rates (7.44%) observed for artificial juvenile broad headed snakes (Hoplocephalus bungaroides; Webb and Whiting 2006) and uropeltid snakes (5%; Cyriac and Kodandaramaiah 2019). Using clay models as a proxy for live animal interactions can have limitations (Bateman et al. 2017), yet we consider our predation attempts to be an accurate representation of at least bird and rodent predation at our study sites, as predation attempts were significantly higher on snake models compared to control balls. The depredated model snakes had strike marks primarily on the head and tail and occasionally had been flipped by an avian predator and pecked on the ventral surface, typical strategies for predators of snakes (Webb and Whiting 2006). It is likely that predation of juvenile tiger snakes at these sites is in reality a rare occurrence and indeed the observed predation rate could be an overestimate as live juvenile snakes spend limited time basking (Webb and Whiting 2005) and artificial snakes do not respond with anti-predator behaviours.
Environmental habitat structure influences the predation risk for reptiles by determining the protective qualities of refuge sites or structural features predators use to hunt (Hawlena et al. 2010; Martin and Murray 2011; Steidl et al. 2013). The considerable habitat structural changes caused by invasive grasses in wetlands can modify trophic interactions sufficiently to impact on overall prey survivorship (DeVore and Maerz 2014). The dominant predators at our study sites are largely visual hunters, thus the artificial snakes were probably more conspicuous in habitats that have reduced structural complexity and increased light availability (Daly et al. 2008; Sato et al. 2014). This is consistent with other studies that found greater predator diversity along open habitat edges (Anderson and Burgin 2008). Birds specifically use elevated perches along habitat edges to forage in open environments (Hansen et al. 2019) and can show a preference for hunting in more homogenous habitats (Hawlena et al. 2010). Our results suggest that kikuyu dominated vegetation is not influencing predation risk for juvenile tiger snakes but riparian vegetation with woody stemmed vegetation and structural complexity provides safer habitats for young tiger snakes, highlighting the value of maintaining sedge and woodland habitats in these urban wetlands.
Thermal quality of vegetation
Kikuyu grass dominated wetland habitats had similar thermal properties to those dominated by natural vegetation. Similarly, Abom et al. (2015) found that the thermal quality within grader grass (Themeda quadrivalvis) did not differ from within native vegetation, offering thermally equivalent shelter sites for a number of skink and snake species. As the thermal environments within these vegetation communities are so similar, despite a change in habitat composition, the way tiger snakes thermoregulate and exploit the thermoregulatory opportunities in these environments has remained unaffected (Cornelis 2021). Not all vertebrate species are disadvantaged by the invasion of exotic grasses (Malo et al. 2012; Lindenmayer et al. 2017); the thermal quality and dense structure provided by kikuyu grass may be particularly suitable for snakes as they can easily move through the vegetation and thermoregulate (McDonald and Luck 2013; Abom et al. 2015). The only notable differences were that riparian woodland habitats provided the most stable thermal environments with the least extreme temperature fluctuations, which is unsurprising given the effects of canopy cover in providing shade and reducing radiation to the sky (Breshears et al. 1998; Yates et al. 2000). In summer in kikuyu dominated communities, riparian woodlands provided the highest proportion of optimal tiger snake temperature compared to other habitats (Table S2). The most challenging temperatures for tiger snakes occur in summer and winter. In Perth during summer, Ta regularly exceeds 40 °C and winter nights can drop below 0 °C (Bureau of Meteorology 2022). Snakes are at considerable risk of exceeding their voluntary thermal maxima (35.5 °C) in summer or critical thermal minima (2 °C) during winter (Shine 1977; Lillywhite 1980; Shine and Mason 2004). In some cases, habitat types in either vegetation community approached these temperatures but the thermal stability, with generally lower maximum and higher minimum temperatures, offered by the woodland habitat types may offer tiger snakes the most valuable thermoregulatory and hibernation opportunities (Table S2).
Management and conservation implications
Seventy percent of urban wetland habitat on the Swan Coastal Plain between Wedge Island and Mandurah Western Australia has been lost to urban development and agriculture (Halse 1989). Their high biodiversity value means that appropriate conservation and management of remaining wetlands is of upmost importance. However, of the remaining 30% of wetlands on the Swan Coastal Plain, only 17% are vegetated with predominantly native vegetation (Hill et al. 1996). Weeds have been identified as a key threat to Perth metropolitan wetlands (Conservation and Parks Commission 2017) and as such weed management and rehabilitation plans have been implemented by many local governments and/or the Department of Biodiversity, Conservation and Attractions. Complete eradication of kikuyu grass and revegetation with native species can be extremely difficult (Department of Environment and Conservation 2012) and may not be successful in some situations. Our work suggests that total eradication of kikuyu grass is not necessary to maintain western tiger snake populations in wetlands of the Swan Coastal Plain and indeed large-scale removal of kikuyu may leave snakes and their prey without shelter sites. We suggest that an alternative to eradication that may improve habitat quality for the snakes and their major prey items would be to increase structural habitat complexity, particularly in areas of homogenous open kikuyu habitat. This could be achieved by removing smaller plots of kikuyu grass and planting more structurally complex native species, with scattered canopy cover. A suitably large area of kikuyu grass would need to be removed to allow for the establishment of these slower growing native species as perennial grasses such are kikuyu can quickly smother native plants (Department of Environment and Conservation 2012), but buffer areas of kikuyu should be left to provide habitat while the native vegetation becomes established. Though this method may result in positive outcomes for local western tiger snake and frog populations, more research is needed to determine how kikuyu dominated vegetation is used by other fauna, and the potential impacts of restoration on other flora and fauna species within the wetland ecosystem.
In Perth, tiger snakes are only known to persist in seven of the potential hundreds of available wetlands (Hill et al. 1996; Lettoof et al. 2021a). Although the exact habitat requirements necessary for supporting tiger snake populations are unknown, we suspect adequate vegetation structure and area plays a vital role. Over-grown kikuyu grass provides the necessary structural features in wetlands where native riparian vegetation no longer exists, providing suitable prey, anti-predator and thermal functions. Future research efforts should focus on assessing differences in habitat characteristics of wetlands with and without tiger snakes to determine the factors that allow tiger snakes to persist in urban wetlands, how tiger snakes utilise the habitats we described in this study (e.g. space-use, movement and habitat selection preferences), and the population size and stability of remnant populations.
Data availability
All relevant raw data and R scripts are available on request from the authors.
References
Abom R, Vogler W, Schwarzkopf L (2015) Mechanisms of the impact of a weed (grader grass, Themeda quadrivalvis) on reptile assemblage structure in a tropical savannah. Biol Cons 191:75–82
Anderson L, Burgin S (2008) Patterns of bird predation on reptiles in small woodland remnant edges in peri-urban north-western Sydney, Australia. Landsc Ecol 23:1039–1047
Aubret F (2004) Aquatic locomotion and behaviour in two disjunct populations of Western Australian tiger snakes, Notchis ater occidentalis. Aust J Zool 52:357–368
Balmford A, Bruner A, Cooper P, Costanza R, Farber S, Green RE, Turner RK (2002) Economic reasons for conserving wild nature. Science 297:950–953
Barton K (2015) Package ‘mumin.’ Version 1:18
Bateman P, Fleming P, Wolfe A (2017) A different kind of ecological modelling: the use of clay model organisms to explore predator–prey interactions in vertebrates. J Zool 301:251–262
Bates D, Maechler M, Bolker B, Walker S (2014) lme4: linear mixed-effects models using Eigen and S4. R package version 1.1–7
Battles AC, Whittle TK, Stehle CM, Johnson MA (2013) Effects of human land use on prey availability and body condition in the green anole lizard. Anol Carol Herpetol Conserv Biol 8:16
Bird MS, Day JA, Rebelo AJ (2013) Physico-chemical impacts of terrestrial alien vegetation on temporary wetlands in a sclerophyllous Sand fynbos ecosystem. Hydrobiologia 711:115–128
Blouin-Demers G, Weatherhead PJ (2002) Habitat-specific behavioural thermoregulation by black rat snakes (Elaphe obsoleta obsolete). Oikos 97:59–68
Boeye J, Kubisch A, Bonte D (2014) Habitat structure mediates spatial segregation and therefore coexistence. Landsc Ecol 29:593–604
Boon M, Tesfamichael S (2017) Wetland vegetation integrity assessment with low altitude multispectral UAV imagery. Int Archivf Photogramm Remote Sens Spatial Info Sc 42:55
Bradshaw CJA, Bowman DMJS, Bond NR, Murphy BP, Moore AD, Fordham DA, Thackway R, Lawes MJ, McCallum H, Gregory SD, Dalal RC, Boer MM, Lynch AJJ, Bradstock RA, Brook BW, Henry BK, Hunt LP, Fisher DO, Hunter D, Johnson CN, Keith DA, Lefroy EC, Penman TD, Meyer WS, Thomson JR, Thornton CM, Vanderwal J, Williams RJ, Keniger L, Specht A (2013) Brave new green world—consequences of a carbon economy for the conservation of Australian biodiversity. Biol Cons 161:71–90
Breshears DD, Nyhan JW, Heil CE, Wilcox BP (1998) Effects of woody plants on microclimate in a semiarid woodland: soil temperature and evaporation in canopy and intercanopy patches. Int J Plant Sci 159(6):1010–1017
Bureau of Meteorology (2022) Climate statistics for Australian locations, Bureau of Meteorology. Australian Government
Burgin S, Franklin MJ, Hull L (2016) Wetland loss in the transition to urbanisation: a case study from Western Sydney, Australia. Wetlands 36:985–994
Burrow A, Maerz J (2022) How plants affect amphibian populations. Biol Rev 97:1749–1767
Catling PM (2005) Effects of invasive alien plants on birds: some examples from North America. Biodiversity 6(3):30–39
Coleman S (2017) Australia state of the environment 2016: built environment. Department of the Environment and Energy
Colman NJ, Gordon CE, Crowther MS, Letnic M (2014) Lethal control of an apex predator has unintended cascading effects on forest mammal assemblages. Proc Royal Soc Biol Sci 281:20133094
Colman NJ, Crowther MS, Letnic M (2015) Macroecological patterns in mammal abundances provide evidence that an apex predator shapes forest ecosystems by suppressing herbivore and mesopredator abundance. J Biogeogr 42:1975–1985
Conservation and Parks Commission (2017) Periodic assessment—Lakes and wetlands of the Perth metropolitan area.
Cook GD, Grice AC (2013) Historical perspectives on invasive grasses and their impact on wildlife in Australia. Wildl Soc Bull 37:469–477. https://doi.org/10.1002/wsb.309
Cooper CE, Withers PC (2004) Patterns of body temperature variation and torpor in the numbat, Myrmecobius fasciatus (Marsupialia: Myrmecobiidae). J Therm Biol 29:277–284
Cornelis J (2021) The serpent in the garden of weeden: a comparison of western tiger snake (Notechis scutatus occidentalis) habitat quality and thermal ecology in native and invasive vegetation. Curtin University, Perth
Cornelis J, Cooper CE, Lettoof DC, Mayer M, Marshall BM (2023) Stuck in the weeds: invasive grasses reduce tiger snake movement. bioRxiv 191:75
Crawley MJ (2007) The R book. Wiley, Chichester
Cresswell I, Murphy H (2017) Australia state of the environment 2016: Biodiversity, independent report to the Australian Government Minister for the Environment and Energy. Australian Government Department of the Environment and Energy, Canberra
Culbert PD, Radeloff VC, Flather CH, Kellndorfer JM, Rittenhouse CD, Pidgeon AM (2013) The influence of vertical and horizontal habitat structure on nationwide patterns of avian biodiversity. Auk 130:656–665
Cyriac VP, Kodandaramaiah U (2019) Conspicuous colours reduce predation rates in fossorial uropeltid snakes. PeerJ 7:e7508
Daly BG, Dickman CR, Crowther MS (2008) Causes of habitat divergence in two species of agamid lizards in arid central Australia. Ecology 89:65–76
Department of Environment and Conservation (2012) Wetland weeds. In: Bell T (ed) A guide to managing and restoring wetlands in Western Australia. Department of Environment and Conservation, Western Australia
DeVore JL, Maerz JC (2014) Grass invasion increases top-down pressure on an amphibian via structurally mediated effects on an intraguild predator. Ecology 95:1724–1730
Doody JS, Soennichsen KF, James H, McHenry C, Clulow S (2021) Ecosystem engineering by deep-nesting monitor lizards. Ecology 102:e03271
Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, Marquéz JRG, Gruber B, Lafourcade B, Leitão PJ (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36:27–46
Douglas MM, O’Connor RA (2003) Effects of the exotic macrophyte, para grass (Urochloa mutica), on benthic and epiphytic macroinvertebrates of a tropical floodplain. Freshw Biol 48:962–971. https://doi.org/10.1046/j.1365-2427.2003.01072.x
Douglas MM, O’Connor RA (2004) Weed invasion changes fuel characteristics: para grass (Urochloa mutica (Forssk.) TQ Nguyen) on a tropical floodplain. Ecol Manag Restor 5(2):143–145
Douglas MM, Setterfield SA, O’Connor RA, Ferdinands K, Rossiter NA, Brooks KJ, Ryan B, Parr C (2006) Different weeds, different habitats, same effects: exotic grass invasion in tropical woodlands and wetlands. Proceedings of the 5th Australian Weeds Conference. Weed Management Society of South Australia Adelaide, South Australia, pp 811–814
Falaschi M, Melotto A, Manenti R, Ficetola GF (2020) Invasive species and amphibian conservation. Herpetologica 76(2):216–227
Faulkner S (2004) Urbanization impacts on the structure and function of forested wetlands. Urban Ecosyst 7:89–106
Ferdinands K, Beggs K, Whitehead P (2005) Biodiversity and invasive grass species: multiple-use or monoculture? Wildl Res 32:447–457
Fox BJ, Fox MD, Taylor JE, Jackson GP, Simpson J, Higgs P, Rebec L, Avery R (1996) Comparison of regeneration following burning, clearing or mineral sand mining at Tomago, NSW: I. Structure and growth of the vegetation. Aust J Ecol 21:184–199
Gaertner M, Richardson DM, Privett SD (2011) Effects of alien plants on ecosystem structure and functioning and implications for restoration: insights from three degraded sites in South African fynbos. Environ Manage 48:57–69
Garden JG, Mcalpine CA, Possingham HP, Jones DN (2007) Habitat structure is more important than vegetation composition for local-level management of native terrestrial reptile and small mammal species living in urban remnants: a case study from Brisbane, Australia. Austral Ecol 32:669–685
Gonzalez F (2009) Socio-environmental dynamics of biological invasions: The case of Kikuyu grass (Pennisetum clandestinum) in the Cotahuasi Valley, southern. ProQuest Dissertations Publishing
Grant PB, Samways MJ (2016) Use of ecoacoustics to determine biodiversity patterns across ecological gradients. Conserv Biol 30(6):1320–1329
Hacking J, Abom R, Schwarzkopf L (2014) Why do lizards avoid weeds? Biol Invasions 16:935–947
Halse S (1989) Wetlands of the swan coastal plain past and present. In: Proceedings of the swan coastal plain groundwater management conference, Water Resources Council, pp 105–112
Hamer AJ, McDonnell MJ (2008) Amphibian ecology and conservation in the urbanising world: a review. Biol Cons 141:2432–2449
Hansen NA, Sato CF, Michael DR, Lindenmayer DB, Driscoll DA (2019) Predation risk for reptiles is highest at remnant edges in agricultural landscapes. J Appl Ecol 56:31–43
Hawlena D, Saltz D, Abramsky Z, Bouskila A (2010) Ecological trap for desert lizards caused by anthropogenic changes in habitat structure that favor predator activity. Conserv Biol 24:803–809
Heard G, Robertson P, Scroggie M (2008) Microhabitat preferences of the endangered growling grass frog Litoria raniformis in southern Victoria. Aust Zool 34:414–425
Hill A, Semeniuk C, Semeniuk V, Del Marco A (1996) Wetland mapping, classification and evaluation. [‘Wetlands of the Swan Coastal Plain, Western Australia’Vol. 2.], Water and Rivers Commission of Western Australia and the Western Australian Department of Environmental Protection, Perth.
Hoskin C, Grigg G, Stewart D, Macdonald S (2015) Frogs of Australia (1.0)[Mobile application software]
Huey RB (1991) Physiological consequences of habitat selection. Am Nat 137:S91–S115
Kassambara A, Mundt F (2020) Factoextra: extract and visualize the results of multivariate data analyses. 2020 R package version 1.0. 7. Google Scholar There is no corresponding record for this reference
Kinzig A, Grove J (2001) Urban-suburban eco-logy, encyclopedia of biodiversity, vol 5. Academic Press, Cambridge
Kuch U, Keogh JS, Weigel J, Smith LA, Mebs D (2005) Phylogeography of Australia’s king brown snake (Pseudechis australis) reveals Pliocene divergence and Pleistocene dispersal of a top predator. Naturwissenschaften 92:121–127
Ladyman M, Bradshaw D (2003) The influence of dehydration on the thermal preferences of the Western tiger snake, Notechis scutatus. Biochem Syst Environ Physiol 173:239–246
Landsman AP, Bowman JL (2017) Discordant response of spider communities to forests disturbed by deer herbivory and changes in prey availability. Ecosphere 8:e01703
Lê S, Josse J, Mazet F (2008) Package ‘FactoMineR. J Stat Softw [internet] 25:1–18
Lee S, Dunn R, Young R, Connolly R, Dale P, Dehayr R, Lemckert C, McKinnon S, Powell B, Teasdale P (2006) Impact of urbanization on coastal wetland structure and function. Aust Ecol 31:149–163
Lemly AD, Kingsford RT, Thompson JR (2000) Irrigated agriculture and wildlife conservation: conflict on a global scale. Environ Manage 25:485–512
Lenth R, Singmann H, Love J, Buerkner P, Herve M (2018) Emmeans: estimated marginal means, aka least-squares means. R Package Version 1:3
Lettoof D, Von Takach B, Bateman PW, Gagnon MM, Aubret F (2020) Investigating the role of urbanisation, wetlands and climatic conditions in nematode parasitism in a large Australian elapid snake. Int J Parasitol Parasites Wildlife 11:32–39
Lettoof D, Santoro A, Swinstead C, Cornelis J (2021a) First record of predation of a hatchling turtle by the Western tiger snake (Notechis scutatus occidentalis). Aust Zool 41:773
Lettoof DC, Cornelis J, Jolly CJ, Aubret F, Gagnon MM, Hyndman TH, Barton DP, Bateman PW (2021b) Metal (loid) pollution, not urbanisation nor parasites predicts low body condition in a wetland bioindicator snake. Environ Pollut 22021:118674
Lettoof DC, Thomson VA, Cornelis J, Bateman PW, Aubret F, Gagnon MM, von Takach B (2022) Bioindicator snake shows genomic signatures of natural and anthropogenic barriers to gene flow. PLoS ONE 16:e0259124
Lillywhite HB (1980) Behavioral thermoregulation in Australian elapid snakes. Copeia 1980:452–458
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Lindenmayer DB, Wood J, MacGregor C, Hobbs RJ, Catford JA (2017) Non-target impacts of weed control on birds, mammals and reptiles. Ecosphere 8(5):e01804. https://doi.org/10.1002/ecs2.1804
Lindsay EA, Cunningham SA (2012) Effects of exotic grass invasion on spatial heterogeneity in the ground-layer of grassy woodlands. Biol Invasions 14:203–213
Litt AR, Steidl RJ (2011) Interactive effects of fire and nonnative plants on small mammals in grasslands: effets Interactifs du Feu et des Plantes non Indigènes sur les Petits Mammifères Dans les Prairies. Wildl Monogr 176:1–31
Liu G, Rowley JJ, Kingsford RT, Callaghan CT (2021) Species’ traits drive amphibian tolerance to anthropogenic habitat modification. Glob Change Biol 27:3120–3132
Lutterschmidt WI, Reinert HK (2012) Modeling body temperature and thermal inertia of large-bodied reptiles: support for water-filled biophysical models in radiotelemetric studies. J Therm Biol 37:282–285
Maerz J, Brown C, Chapin C, Blossey B (2005) Can secondary compounds of an invasive plant affect larval amphibians? Funct Ecol 19:970–975
Malo AF, Godsall B, Prebble C, Grange Z, McCandless S, Taylor A, Coulson T (2012) Positive effects of an invasive shrub on aggregation and abundance of a native small rodent. Behav Ecol 21:759–767
Martin LJ, Murray BR (2011) A predictive framework and review of the ecological impacts of exotic plant invasions on reptiles and amphibians. Biol Rev 86:407–419
McCauley DJ, Keesing F, Young TP, Allan BF, Pringle RM (2006) Indirect effects of large herbivores on snakes in an African savanna. Ecology 87:2657–2663
McDonald PJ, Luck GW (2013) Density of an environmental weed predicts the occurrence of the king brown snake (Pseudechis australis) in central Australia. Herpetol J 23:161–165
McDonnell MJ, Pickett ST, Pouyat RV (1993) The application of the ecological gradient paradigm to the study of urban effects. In: McDonnell MJ, Pickett STA (eds) Humans as components of ecosystems. Springer, New York, pp 175–189
Naimi B (2015) Usdm: uncertainty analysis for species distribution models. R package version 1.1–15. R Documentation http://www.rdocumentation.org/packages/usdm
Nicholls RJ, Hoozemans FM, Marchand M (1999) Increasing flood risk and wetland losses due to global sea-level rise: regional and global analyses. Glob Environ Chang 9:S69–S87
Norbury G, van Overmeire W (2019) Low structural complexity of nonnative grassland habitat exposes prey to higher predation. Ecol Appl 29:e01830
Nordberg EJ, Schwarzkopf L (2019) Predation risk is a function of alternative prey availability rather than predator abundance in a tropical savanna woodland ecosystem. Sci Rep 9:1–11
Nowakowski AJ, Thompson ME, Donnelly MA, Todd BD (2017) Amphibian sensitivity to habitat modification is associated with population trends and species traits. Glob Ecol Biogeogr 26:700–712
Pianka ER (1986) Ecology and natural history of desert lizards. Princeton University Press
Read JL, Scoleri V (2015) Ecological implications of reptile mesopredator release in arid South Australia. J Herpetol 49:64–69
Revelle W (2015) Package ‘psych.’ Compr R Archiv Netw 337:338
Rossiter NA, Setterfield SA, Douglas MM, Hutley LB (2003) Testing the grass-fire cycle: alien grass invasion in the tropical savannas of northern Australia. Divers Distrib 9:169–176. https://doi.org/10.1046/j.1472-4642.2003.00020.x
Roznik E, Reichling S (2021) Survival, movements and habitat use of captive-bred and reintroduced dusky gopher frogs. Anim Conserv 24:51–63
RStudio Team (2020) RStudio: integrated development for R. RStudio Inc, Boston
Sammon JG, Wilkins KT (2005) Effects of an invasive grass (Bothriochloa ischaemum) on a grassland rodent community. Tex J Sci 57(4):371–383
Sato CF, Wood JT, Schroder M, Green K, Osborne WS, Michael DR, Lindenmayer DB (2014) An experiment to test key hypotheses of the drivers of reptile distribution in subalpine ski resorts. J Appl Ecol 51:13–22
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Schweiger AK, Cavender-Bares J, Townsend PA, Hobbie SE, Madritch MD, Wang R, Tilman D, Gamon JA (2018) Plant spectral diversity integrates functional and phylogenetic components of biodiversity and predicts ecosystem function. Nat Ecol Evol 2:976–982
Seebacher F, Shine R (2004) Evaluating thermoregulation in reptiles: the fallacy of the inappropriately applied method. Physiol Biochem Zool 77:688–695
Sergio F, Caro T, Brown D, Clucas B, Hunter J, Ketchum J, McHugh K, Hiraldo F (2008) Top predators as conservation tools: ecological rationale, assumptions and efficacy. Annu Rev Ecol Evol Syst 39:1–19
Shine R (1977) Reproduction in Australian elapid snakes II. Female reproductive cycles. Aust J Zool 25:655–666
Shine R, Mason RT (2004) Patterns of mortality in a cold-climate population of garter snakes (Thamnophis sirtalis parietalis). Biol Cons 120(2):201–210
Sieben EJJ, Collins NB, Corry FTJ, Kotze DC, Job N, Muasya AM, Venter CE, Mtshali H, Zondo SA, Janks M, Pretorius L (2016) The vegetation of grass lawn wetlands of floodplains and pans in semi-arid regions of South Africa: description, classification and explanatory environmental factors. S Afr J Bot 104:215–224
Skaug H, Nielsen A, Magnusson A, Bolker B (2013) Glmmadmb package (0.6. 7.1 edn). http://glmmadmb.r-forge.r-project.org
Skórka P, Lenda M, Tryjanowski P (2010) Invasive alien goldenrods negatively affect grassland bird communities in Eastern Europe. Biol Cons 143(4):856–861
Štajerová K, Šmilauer P, Brůna J, Pyšek P (2017) Distribution of invasive plants in urban environment is strongly spatially structured. Landsc Ecol 32:681–692
Stanton RA, Boone WW, Soto-Shoender J, Fletcher RJ, Blaum N, McCleery RA (2018) Shrub encroachment and vertebrate diversity: a global meta-analysis. Global Ecol Biogeogr 27:368–379. https://doi.org/10.1111/geb.12675
Steidl RJ, Litt AR, Matter WJ (2013) Effects of plant invasions on wildlife in desert grasslands. Wildl Soc Bull 37:527–536
Stein A, Gerstner K, Kreft H (2014) Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol Lett 17:866–880
Stephens CJ, Facelli JM, Austin AD (2008) The impact of bridal creeper (Asparagus asparagoides) on native ground-cover plant diversity and habitat structure. Plant Prot Q 23(3):136–143
Valentine LE, Roberts B, Schwarzkopf L (2007) Mechanisms driving avoidance of non-native plants by lizards. J Appl Ecol 44:228–237
Vences M, Chiari Y, Teschke M, Randrianiaina RD, Raharivololoniaina L, Bora P, Vieites DR, Glaw F (2008) Which frogs are out there? A preliminary evaluation of survey techniques and identification reliability of Malagasy amphibians. In: A conservation strategy for the amphibians of Madagascar, Museo Regionale di Scienze Naturali, pp 233–252
Virkki DA, Tran C, Castley JG (2012) Reptile responses to lantana management in a wet sclerophyll forest, Australia. J Herpetol 46:177–185
von Takach B, Scheele BC, Moore H, Murphy BP, Banks SC (2020) Patterns of niche contraction identify vital refuge areas for declining mammals. Divers Distrib 26:1467–1482
von Takach B, Jolly CJ, Dixon KM, Penton CE, Doherty TS, Banks SC (2022) Long-unburnt habitat is critical for the conservation of threatened vertebrates across Australia. Landsc Ecol 37:1–14
Webb JK, Whiting MJ (2005) Why don’t small snakes bask? Juvenile broad-headed snakes trade thermal benefits for safety. Oikos 110:515–522
Webb JK, Whiting MJ (2006) Does rock disturbance by superb lyrebirds (Menura novaehollandiae) influence habitat selection by juvenile snakes? Austral Ecol 31:58–67
Webb JK, Shine R, Pringle RM (2005) Canopy removal restores habitat quality for an endangered snake in a fire suppressed landscape. Copeia 2005:894–900
Yates CJ, Norton DA, Hobbs RJ (2000) Grazing effects on plant cover, soil and microclimate in fragmented woodlands in south-western Australia: implications for restoration. Aust Ecol 25(1):36–47
Zavaleta ES, Hobbs RJ, Mooney HA (2001) Viewing invasive species removal in a whole-ecosystem context. Trends Ecol Evol 16:454–459
Zedler JB, Kercher S (2004) Causes and consequences of invasive plants in wetlands: opportunities, opportunists and outcomes. Crit Rev Plant Sci 23:431–452
Zipkin EF, DiRenzo GV, Ray JM, Rossman S, Lips KR (2020) Tropical snake diversity collapses after widespread amphibian loss. Science 367:814–816
Acknowledgements
This study was supported by a grant from the Holsworth Wildlife Research Endowment to DCL. BvT acknowledges the support of the Forrest Research Foundation. The authors thank Aleesha Turner, Serin Subaraj and Brae Price for their assistance in the field. Experiments followed the Australian Code of Practice for the care and use of animals for scientific purposes and were approved by the Curtin University Animal Ethics committee (ARE2019-24). We also thank the staff at the Department of Biodiversity Conservation and Attractions and the staff at Regional Parks for allowing us access to the study sites and issuing the required permits.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Jari Cornelis received funding from Curtin University for this research and Damian Lettoof further contributed funding from the Holsworth Wildlife Research Endowment, https://www.ecolsoc.org.au/awards/holsworth/. B. von Takach acknowledges the support of the Forrest Research Foundation.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by JC, BvT and DL. The first draft of the manuscript was written by JC is and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Additional funding was provided by the Holsworth Wildlife Research Endowment via DCL.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cornelis, J., von Takach, B., Cooper, C.E. et al. Quantifying the impacts of an invasive weed on habitat quality and prey availability for tiger snakes (Notechis scutatus) in urban wetlands. Wetlands Ecol Manage 31, 715–732 (2023). https://doi.org/10.1007/s11273-023-09943-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-023-09943-2