Abstract
Wetlands support unique biodiversity and play a key role in carbon cycles, but have dramatically declined in extent worldwide. Restoration is imperative yet often challenging to counteract loss of functions. Nature-based solutions such as the creation of novel ecosystems may be an alternative restoration approach. Targeted restoration strategies that account for the effects of vegetation on greenhouse gas (GHG) fluxes can accelerate the carbon sink function of such systems. We studied the relationships between vegetation, bare soil, and GHG dynamics on Marker Wadden in the Netherlands, a newly-created 700-ha freshwater wetland archipelago created for nature and recreation. We measured CO2 and CH4 fluxes, and soil microbial activity, in three-year-old soils on vegetated, with distinct species, and adjacent bare plots. Our results show that CH4 fluxes positively related to organic matter and interacted between organic matter and water table in bare soils, while CH4 fluxes positively related to plant cover in vegetated plots. Similarly, Reco in bare plots negatively related to water table, but only related positively to plant cover in vegetated plots, without differences between vegetation types. Soil microbial activity was higher in vegetated soils than bare ones, but was unaffected by substrate type. We conclude that GHG exchange of this newly-created wetland is controlled by water table and organic matter on bare soils, but the effect of vegetation is more important yet not species-specific. Our results highlight that the soil and its microbial community are still young and no functional differentiation has taken place yet and warrants longer-term monitoring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wetlands are iconic and important ecosystems that support unique biodiversity (Denny 1994; Wiegleb et al. 2017) and play a crucial role in the global carbon and water cycle (Battin et al. 2009; Oertel et al. 2016; Temmink et al. 2022a; Vörösmarty and Sahagian 2000). However, natural wetlands and the ecosystem services they provide have been degrading at a rapid pace (Gupta et al. 2020; Yang et al. 2020). Recent estimates suggest a loss of c. 20% of the global inland wetland area over 300 years. Moreover, the highest losses have been for the USA and China (40–50%) and in Europe (70–90%) (Fluet-Chouinard et al. 2023). As such, restoration is an important tool to counteract the loss of biodiversity (Erwin 2009; Meli et al. 2014). However, ecological restoration of wetlands, which hitherto largely has focused on returning degraded wetlands to pre-human-impact conditions, is often challenging (Higgs et al. 2018). To overcome this challenge, forward-looking restoration approaches such as the creation of new ecosystems in a human-influenced landscape are nowadays often adopted (Hobbs et al. 2009, 2006; Kentula 1996; Temmink et al. 2022b; van Leeuwen et al. 2021).
An important aim of wetland restoration is to bring back the ability of these ecosystems to store carbon in biomass and the soil (Temmink et al. 2022a). In natural wetlands, anoxic soil conditions related to a high water table slow down the decomposition of organic matter, which can result in long-term carbon storage (Kayranli et al. 2010). However, wetlands can emit carbon in the form of methane (CH4), which is a more potent GHG than carbon dioxide (CO2) especially in the short-term (GHG) than carbon dioxide (CO2) (IPCC 2021). Next to water table dynamics, vegetation can strongly control carbon dynamics (Hobbs et al. 2006; Temmink et al. 2022b; Whiting and Chanton 1993). For example, species showing much aerenchyma such as Phragmites australis and Typha latifolia can act as a chimney for CH4, bypassing the oxygen-rich top layer of the sediment where CH4 oxidation takes place, leading to direct CH4 release from the soil to the atmosphere (Dingemans et al. 2011; Vroom et al. 2022). Similarly, oxygen transport by aerenchymous plants can increase CH4 oxidation and thus reduce CH4 emission (Armstrong 1980; Colmer 2003). Furthermore, vegetation can affect the microbial composition of the soil via radial oxygen loss (Armstrong et al. 1999; Sasikala et al. 2009) and input of root exudates (Panchal et al. 2022). The dominant microbial community, shaped by oxygen levels in the soil, determines the balance in CO2 and CH4 production (Sutton-Grier and Megonigal 2011). Therefore, the development of both vegetation and microbial community is strongly linked to GHG fluxes in wetlands (Robroek et al. 2015; Turner et al. 2020). In light of climate change, it is therefore important to define clear goals with respect to water level and vegetation type when creating new ecosystems (IPCC Report 2022), as it can determine whether a new ecosystem turns into a carbon source or sink (Audet et al. 2013; Erwin 2009).
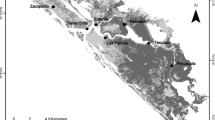
An iconic example of a novel ecosystem with a forward-looking approach is Marker Wadden archipelago in a freshwater lake (Lake Markermeer) in the Netherlands that formed after closing off a marine estuary (van Leeuwen et al. 2021) (Fig. 1). As a consequence of cutting of the marine connection, the ecological value of the newly formed Lake Markermeer declined rapidly. Wind driven sediment resuspension in the closed lake resulted in very turbid water, leading to low productivity and biodiversity. A classical ecological restoration approach was not feasible from a socio-economical perspective (Gulati and Donk 2002; van Leeuwen et al. 2021). Alternatively, a large wetland archipelago was designed to improve water clarity and biodiversity, and increase the heterogeneity of the lake (van Leeuwen et al. 2021; Verschoor and Rijsdorp 2012). In such novel wetlands, it however remains unclear what the effect of developing vegetation and soil microbial community are on GHG dynamics.
This study aims to unravel the effect of the developing vegetation and microbial community on GHG fluxes in the newly created 700-ha Marker Wadden wetland archipelago. We performed a field study to quantify GHG fluxes and soil microbial activity in plots dominated by various wetland vegetation and compared these to adjacent bare soil. We hypothesized that (i) net ecosystem exchange (NEE) would be reduced (i.e., net carbon uptake) in vegetated soil compared to bare soil, due to photosynthesis being higher than respiration, (ii) some species, characterized by aerenchyma tissue, like Phragmites australis and Typha latifolia, have higher CH4 emission compared to other species, (iii) that the microbial activity of the soil differs per species, because of the supply of readily-decomposable carbon (dead organic material and root exudates) that can be used as a carbon substrate for microbes, and (iv) increased soil activity results in enhanced GHG fluxes. The outcome will be discussed in relation to the functioning of novel wetlands with respect to their carbon sequestration capacity and GHG dynamics.
Materials and methods
Study site
Our study site was located in Lake Markermeer in the Netherlands, which was formerly part of the inland sea Zuiderzee, but was closed off from the Wadden Sea by the construction of two dykes (Afsluitdijk and Houtribdijk) [Fig. 1A, for more information, see van Leeuwen et al. (2022)]. This converted the system from a marine estuary to a shallow and turbid freshwater lake. Ecological values including biodiversity have gradually declined over time (Cremer et al. 2009; Noordhuis 2014; van Leeuwen et al. 2022). To improve the ecological state of Lake Markermeer, the Dutch Society for Nature Conservation (‘Natuurmonumenten’) started a forward-looking restoration project. A 700-ha human-made archipelago named Marker Wadden was constructed between 2016 and 2020 to create shelter and gradual land-to-water transitions (Fig. 1B, 52°35′30″N 5°22′43″E). The wetlands were created by first constructing ring dikes of sand, which were filled up with Holocene marine clay and silt that was pumped from the bottom of the lake up to a depth of –20 m (Saaltink et al. 2016; van Leeuwen et al. 2021). The elevation of Marker Wadden lies between c. 0 (marsh) and 1.5 m (sandy walking paths) N.A.P (c. mean sea level). Soil formation started after construction and typically entailed enrichment with organic matter, and, depending on vegetation type, a thick organic layer. The water level of Lake Markermeer is managed and protected from future sea level rise by the Afsluitdijk (i.e., a dam between the sea—both Wadden and indirectly the North Sea and the freshwater lakes). For more information regarding the aims and ecological concept of Marker Wadden, the effects of herbivory, shelter, or primary production in the aquatic system, see van Leeuwen et al. (2021); Saaltink et al. (2019), Jin et al. (2022), and Temmink et al. (2022b).
The Marker Wadden archipelago. a Its location (red box) in the Netherlands (inset, blue) with the two major dykes shaping the current system (Afsluitdijk and Houtribdijk); b the newly constructed archipelago Marker Wadden with soft-sediment wetlands (green); c locations of the sampled plots. Maps were created with Natural Earth (a) and with OpenStreetMap (b-c)
Study design
To study the effect of vegetation type (vegetation for brevity) on GHG fluxes, 32 vegetated and 32 bare plots of approximately 1 m2 were sampled. All vegetated plots consisted of one (n = 19 plots) or two (n = 2 plots) dominant plant species. The dominant species were Cotula coronopifolia, Epilobium hirsutum, Phragmites australis, Rumex hydrolapathum, Rumex maritimus, and Typha latifolia (n = 7 for R. maritimus and n = 5 for the other species). At 0.5–1 m distance from a vegetated plot, a bare plot with visually similar abiotic conditions was selected. To minimize differences in soil type and time since construction (3 years), all plots were selected on the same island (Fig. 1C) and were sampled between July 21st and 23rd in 2020. The days (09.00–17.00 h) were comparable in temperature (average temperature day 1: 18.5 °C, day 2: 17.75 °C, day 3: 21.25 °C), and precipitation (0.0 mm on all days). All measurements were conducted in full sunlight with a clear sky (day 1: 09.00–17.00 h, day 2: 08.00–15.00 h, day 3: 09.00–12.00 h and 14.00–17.00 h).
Greenhouse gas measurements
We measured CO2 and CH4 fluxes in all plots using closed transparent and opaque PVC chambers (diameter 50 cm, height depending on the vegetation: 25–200 cm, fitted with a circulating fan), which were placed on the sediment as such that an airtight seal was established. Chamber height could be adjusted by opaque open top rings where the closed top chambers could sit on. All connections were airtight. The chamber was connected to a LI-7810 Trace Gas Analyzer (LI-COR Biosciences GmbH, Bad Homburg, Germany) with TPE-U(PU) tubes (4 cm inner diameter) via two gas-tight ports in a closed loop. For inundated plots, a floating chamber (30 cm diameter, height 17 cm) was used. Measurements lasted for c. 180 s and included only diffusive fluxes (i.e., no ebullition) (Oliveira Junior et al. 2019). Gas fluxes in each plot were first measured with the transparent chamber to determine Net ecosystem CO2 exchange (NEE) and then with an opaque chamber to calculate ecosystem respiration (Reco). Light and dark measurements of CH4 fluxes were averaged, since they did not differ significantly. In the rare case of an abrupt increase in gas concentration, indicating ebullition, we removed the chamber, vented and replaced it (van Bergen et al. 2019). The differences in CO2 and CH4 fluxes between the vegetated plots and the corresponding bare plots were used to calculate the effect of the vegetation on the fluxes.
Vegetation, soil, and water table measurements
We determined total coverage of vegetation (%), species, cover per species (%), and maximum species height (cm) in each vegetated plot. After GHG measurements, we collected a soil sample in all plots from the top 10 cm, to assess organic matter content and soil microbial activity. Samples were stored in the dark at 4 °C until further analysis. To measure the groundwater level, we dug a 10–40 cm hole and let the water table stabilize for 30 min, after which we measured the water level relative to the soil surface with a ruler. In case the soil was inundated, we measured the depth from the sediment to the water surface.
Chemical analyses and ecophysiological profiling of the microbial community
Wet bulk density was determined by filling aluminum cups with soil and dividing the soil weight by the volume of the cups (38.8 ml). To determine water content and organic matter content of the soil, subsamples were first dried at 70 °C to constant weight, after which the dried samples were ignited at 450 °C for six hours, and remeasured afterward. The soil microbial activity and physiological soil profile were determined with MicroResp™ following Campbell et al. (2003) and Renault et al. (2013). First, all soils were acclimated to room temperature for 3–5 days. We ascertained that the water content of the soils (dry-weight/fresh-weight ratio) fell within the 30–60% moisture range, following prior analysis (see above). To remove stones and large pieces of organic matter, all samples were sieved with a 2 mm sieve, after which deep-well plates were filled with approximately 400 µl soil per well. To assess the physiological profile of the soil, either demineralized water or 30 mg substrate per gram of soil water with different complexities was added. We added, in increasing order of complexity, fructose, glucose, and trehalose, and as a control demineralized water (n = 4 replicates per combination of soil and substrate) (Creamer et al. 2016). The amount of added substrate was standardized to 30 mg per gram of soil water. After substrate addition, colorimetric gel detection plates were filled with a combination of agar and a cresol red indicator solution (cresol red (18.5 mg l−1), potassium chloride (17 g l−1), and sodium bicarbonate (0.315 g l−1). The plates were stored in a dark desiccator for at least two days before the addition of soils and substrates, with self-indicating soda lime that absorbs carbon dioxide (CO2) from the air and changes color as it becomes saturated, and a beaker of water to prevent the agar from drying out.
Soil was added to the deep-well plates the day before the actual incubation and stored at room temperature with an airtight seal to avoid evaporation of soil moisture. At the start of the incubation (t = 0), detection plates were read with a Spark® Multimode Microplate Reader (TECAN, Switzerland) at 570 nm. Only plates with less than a 5% Coefficient of Variance (%CoV) between the wells were used for analyses. Then, the carbon substrate solutions were added to the soil in the deep-well plates using a syringe. Immediately, the filled deep-well and detection plates were placed on top of each other and sealed airtight using a MicroResp™ rubber seal and clamp. After incubating the plates at 25 °C for 6 h, the detection plates were measured again with the Microplate Reader. The CO2 respiration rates (μg g−1 h−1) were calculated using the difference in light absorption between the two measurements (Campbell et al. 2003; Creamer et al. 2016; Renault et al. 2013).
Statistical analyses
All statistical analyses were performed with RStudio version 4.0.3. Prior to analyses, variables CH4 fluxes, plant cover percentage, soil OM content, and CO2 production rate were log-transformed and Reco and CO2 production rates (MicroResp™) were square-root transformed to meet model assumptions (normality of model residuals and variance homogeneity, visually assessed using residual plots). To assess the effect of vegetation presence, we performed paired samples t-tests for CH4 fluxes, Reco, OM content, and CO2 production rate and a Wilcoxon signed rank test for NEE. To test the effect of environmental variables, data from bare plots and vegetated plots were separated. Then, linear models were created with CH4 fluxes, NEE, or Reco as a dependent variable, water table and OM content, and their interaction as independent variables. For vegetated plots, dominant plant species and plant cover percentage were added as additional independent variables. CO2 production rates (i.e., MicroResp analyses) were compared in a linear model using only substrate for bare soils, and adding dominant plant species and their interaction with vegetated soils, as independent variables. Optimal models were chosen using backward selection based on lowest AIC value. Pair-wise differences between dominant plant species were tested using Tukey post-hoc tests if the main effect was significant. Values are reported as mean ± standard error and differences were deemed significant at p < 0.05.
Results
Effects of OM and water table on GHG exchange overruled by plant presence
Soil OM content ranged between 0.5 and 28.7% (average 5.1 ± 0.1%) and did not differ between bare and vegetated plots (t = 0.24, p = 0.809). The water table across all plots ranged from − 35 to 25 cm. NEE was positive in bare plots (i.e., emission to the atmosphere, 18.3 ± 0.8 g CO2 m−2 d−1) and negative—and much lower (V = 528, p < 0.001)—in vegetated plots (− 54.3 ± 1.5 g CO2 m−2 d−1) due to plant CO2 uptake (Fig. 2A). In bare plots, NEE was negatively related to the water table (t = − 4.78, p < 0.001) (Fig. 3A). Contrastingly, there was no effect of water table on NEE in vegetated plots (t = 1.23, p = 0.230) (Fig. 3B). However, T. latifolia had a significantly lower NEE (− 115.8 ± 15.7 g CO2 m−2 d−1) than all other dominant species (t = 2.51, p = 0.02), with a significant effect of cover percentage (t = − 3.25, p = 0.004), indicating that higher cover percentages lead to lower NEE for T. latifolia only.
Impacts of vegetation type on NEE, Reco, and CH4 fluxes. A–C NEE, Reco, and CH4 fluxes for bare and vagetated plots (n = 32) and D–F the calculated impact (difference between bare and vegetated soils) of dominant vegetation on GHG fluxes (n = 16). A negative NEE denotes carbon uptake. Boxplots show the median (middle line), quartiles (boxes), 1.5 times the interquartile range (IQR) (whiskers), and the individual data values (dots). Dots outside the whiskers are extreme values. Significance: *** is p < 0.001; ** is p = 0.001–0.05; species did not show significance pairwise differences
Relationships between OM, water table and plant cover, and GHG fluxes. A Relation between NEE (g CO2 m−2 d−1) and water level (cm) in bare plots (n = 32) and B in vegetated plots (n = 32); C relation between Reco (g CO2 m−2 d−1) and water table (cm) in bare plots (n = 32) and D between Reco (g CO2 m−2 d−1) and plant cover (%) in vegetated plots (n = 32); E relation between CH4 (g CH4 m−2 d−1) and organic matter (%) with interaction with water table (cm) (n = 32) and F Relation between CH4 flux (g CH4 m−2 d−1) and plant cover (%) of vegetated plots (n = 32)
Reco was nearly twice as high in vegetated (34.0 ± 0.7 g CO2 m−2 d−1) as compared to bare plots (18.3 ± 0.8 g CO2 m−2 d−1) (t = 4.48, p < 0.001) (Fig. 2B). Similar to NEE, Reco was negatively related to water table in bare plots (t = − 7.47, p < 0.001) (Fig. 3C). In vegetated plots, we did not find a relationship between Reco and water table. Instead, Reco was positively related to plant cover (F = 19.69, p < 0.001) (Fig. 3D), and while we observed an effect of dominant plant species (F = 6.44, p < 0.001) there were no clear pairwise differences between plant species.
CH4 fluxes were highly variable and ranged from 0.2 to 699.7 mg CH4 m−2 d−1. On average, CH4 fluxes were twice as high in vegetated plots (55.1 ± 4.1 mg CH4 m−2 d−1) as compared to bare plots (25.8 ± 1.8 mg CH4 m−2 d−1) (t = 3.59, p = 0.001) (Fig. 2C). In bare plots, we found a positive relationship between OM content and CH4 fluxes (t = 2.66, p = 0.013); the effect of OM content seemed to depend on water table (t = − 2.86, p = 0.008) (Fig. 3E). In vegetated plots, CH4 fluxes were only positively related to plant cover percentage (t = 2.51, p = 0.018) (Fig. 3F).
The effect of vegetation on soil microbial activity
CO2 production rates measured in the MicroResp analyses were on average slightly higher in soils from vegetated plots (0.465 ± 0.003 µg g−1 h−1) than from bare soils (0.387 ± 0.003 µg g−1 h−1) (t = 2.02, p = 0.045) (Fig. 4). We found no differences in CO2 production between different substrates in bare soils (F = 1.595, p = 0.193) or in vegetated soils (F = 1.42, p = 0.240). In vegetated soils, dominant plant species affected CO2 production (F = 2.93, p = 0.007); specifically soils vegetated by T. latifolia had higher CO2 production than soil with C. coronopifolia (p = 0.035) or P. australis (p = 0.015).
Impacts of substrate type and vegetation type on soil activity. The CO2 production rates for different vegetation types or bare soil with added substrate of increasing complexities (fructose, glucose, and trehalose; water acted as a control) were measured with the MicroResp™ method. Boxplots show the median (middle line), quartiles (boxes), 1.5 times the interquartile range (IQR) (whiskers), and the individual data values (dots). Different letters show significant differences between vegetation
Discussion
Vegetation, but not OM, alters CO2 exchange
Our results reveal that NEE and Reco were negatively related to water level, while plant cover overruled this relationship for Reco. While Reco was also determined by the dominant plant species, this was not the case for NEE. The negative relationship between NEE and water level on bare soils is in line with results obtained in a mesocosm experiment using soils from the same site (Temmink et al. 2021). Indeed, it is known that higher water levels decrease oxygen availability in the soil, which limits soil respiration, resulting in lower CO2 fluxes to the atmosphere (Evans et al. 2021; Schaufler et al. 2010). Furthermore, we expected that higher OM content of the soil would positively relate to Reco, because of higher substrate availability for decomposition (Battin et al. 2009; Langevelda et al. 1997; Schaufler et al. 2010). However, we have not found such a relationship, which may be explained by a non-developed microbial community (see below) or the recalcitrant nature of the organic matter (Inglett et al. 2011). Additionally, we found no difference in soil OM content between bare and vegetated plots, which can be explained by the limited age of the vegetation, limiting the availability of dead organic material input. Therefore, the increase in Reco in vegetated plots is likely explained by the respiration of the plants themselves (and no increase in soil respiration).
OM, water table, and vegetation control CH4 emissions
In our study, CH4 emissions from the soil were highly variable in both the bare and vegetated plots and ranged from 0.01 and 699 mg m−2 d−1. Furthermore, CH4 fluxes were positively related to OM and its interaction with water table in bare soils, but CH4 fluxes were only related to plant cover and were an order of magnitude higher in vegetated plots. At the same site, Temmink et al. (2022b) found higher CH4 fluxes in plots dominated by P. australis than on bare soils (~ 150 mg CH4 m−2 d−1 compared to ~ 1 mg m−2 d−1 on bare soil). It is known that P. australis can both enhance or decrease CH4 emission depending on the balance between O2 transport to the soil, aerenchymous CH4 transport to the atmosphere, and the contribution of additional easily degradable organic material (e.g., root exudates) (Armstrong et al. 1992; van den Berg et al. 2020; Vroom et al. 2022). Furthermore, in our study water level alone had no relationship with CH4 emissions, but showed an interaction with OM in bare soils, which is in line with the mesocosm experiment using soil from our study site (Temmink et al. 2021). In general, they measured CH4 emissions of 6.9 ± 6 mg m−2 d−1 and found a positive effect of water level on CH4 emission. High water levels are known to stimulate CH4 emissions provided that sufficient labile OM is available for methanogenesis (Battin et al. 2009). However, the presence of sulphate as a more favorable terminal electron acceptor is known to inhibit CH4 production and emission (Badiou et al. 2011). In our study area, sulphate is readily available due to the marine origin of the soil (Saaltink et al. 2019), and can thus lower CH4 production and ultimately emission.
Non-developed microbial soil community
Soil productivity measured with MicroResp™ showed that CO2 production from vegetated plots was higher than from bare soils, which is in line with the Reco results. However, there were no differences in CO2 production between substrates in bare soils or vegetated soil, suggesting that the higher Reco was related to direct vegetation effects including plant respiration or ROL. In vegetated soils, T. latifolia had higher CO2 production than C. coronopifolia and P. australis. Similar soil activities in soils with differing vegetation may be explained by the age of the soil of this newly-created wetland. In relatively young soils, the microbial communities are often not well-developed or specialized (Urakawa and Bernhard 2017). Interestingly, the addition of carbon substrates with increasing complexity did not affect the production of CO2, which can indicate that (i) the soils had abundant carbon and that the activity of the soil was not limited by the available organic carbon in the soil (Schaufler et al. 2010) or (ii) that the young microbial community, due to the recent construction of the wetland, was not able to utilize the added substrate (Inglett et al. 2011). We expect that the microbial community will develop and diversify during the succession of the newly established wetlands (Yu et al. 2017). In this light, we expect that the further accumulation of plant material and the loss of the exudates will increase and will shape microbial communities (Morriën et al. 2017; Yang et al. 2020).
Conclusions and implications
Overall, this research shows that vegetation type and water table play an important role in carbon dynamics in the newly constructed wetland Marker Wadden and may determine whether such a soft-sediment wetland develops into a carbon source or a sink in the long term. Specifically, we found that water level is the main driver of CO2 emission in bare soils and plant cover strongly influenced Reco. Furthermore, soil microbial activity of these relatively youngly-developed soils was unaffected by the addition of substrate of increasing complexity. Our results highlight that GHG fluxes are related to vegetation, OM, and water table. To determine whether the Marker Wadden can become a carbon sink annual or multi-annual GHG time series or repeated soil inventories are needed. Therefore, it shows the importance of the management regarding e.g. hydrology and vegetation state, which we showed can have a large effect on the carbon balance of such newly-created wetlands. However, it remains unknown how management choices can control the vegetation to create a landscape that functions as a carbon sink in the long term. Such knowledge can aid to minimize global climate change and wetland degradation as described by the United Nations Decade on Ecosystem Restoration (2021–2030) and the European Green Deal. To design a management plan to do so, it is key to understand the relationship between vegetation, soil activity, and GHG emissions in a well-developed novel wetland.
Data availability
Data is available through Zenodo: https://doi.org/10.5281/zenodo.8123992 (Tak et al. 2023).
References
Armstrong W (1980) Aeration in higher plants. Adv Bot Res 7:225–332. https://doi.org/10.1016/S0065-2296(08)60089-0
Armstrong J, Armstrong W, Beckett PM (1992) Phragmites australis: venturi-and humidity-induced pressure flows enhance rhizome aeration and rhizosphere oxidation. New Phytol 120:197–207
Armstrong J, Afreen-Zobayed F, Blyth S, Armstrong W (1999) Phragmites australis: effects of shoot submergence on seedling growth and survival and radial oxygen loss from roots. Aquat Bot 64:275–289. https://doi.org/10.1016/S0304-3770(99)00056-X
Audet J, Elsgaard L, Kjaergaard C, Larsen SE, Hoffmann CC (2013) Greenhouse gas emissions from a Danish riparian wetland before and after restoration. Ecol Eng 57:170–182. https://doi.org/10.1016/j.ecoleng.2013.04.021
Badiou P, McDougal R, Pennock D, Clark B (2011) Greenhouse gas emissions and carbon sequestration potential in restored wetlands of the Canadian prairie pothole region. Wetl Ecol Manag 19:237–256. https://doi.org/10.1007/s11273-011-9214-6
Battin TJ, Luyssaert S, Kaplan LA, Aufdenkampe AK, Richter A, Tranvik LJ (2009) The boundless carbon cycle. Nat Geosci 2:598–600. https://doi.org/10.1038/ngeo618
Campbell CD, Chapman SJ, Cameron CM, Davidson MS, Potts JM (2003) A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl Environ Microbiol 69:3593–3599. https://doi.org/10.1128/AEM.69.6.3593-3599.2003
Colmer TD (2003) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ 26:17–36. https://doi.org/10.1046/J.1365-3040.2003.00846.X
Creamer RE, Stone D, Berry P, Kuiper I (2016) Measuring respiration profiles of soil microbial communities across Europe using MicroResp™ method. Appl Soil Ecol 97:36–43. https://doi.org/10.1016/j.apsoil.2015.08.004
Cremer H, Bunnik FPM, Kirilova EP, Lammens EHRR, Lotter AF (2009) Diatom-inferred trophic history of IJsselmeer (The Netherlands). Hydrobiologia 631:279–287. https://doi.org/10.1007/s10750-009-9816-7
Denny P (1994) Biodiversity and wetlands. Wetl Ecol Manag 3:55–61
Dingemans BJJ, Bakker ES, Bodelier PLE (2011) Aquatic herbivores facilitate the emission of methane from wetlands. Ecology 92:1166–1173
Erwin KL (2009) Wetlands and global climate change: The role of wetland restoration in a changing world. Wetl Ecol Manag 17:71–84. https://doi.org/10.1007/s11273-008-9119-1
Evans CD, Peacock M, Baird AJ, Artz RRE, Burden A, Callaghan N, Chapman PJ, Cooper HM, Coyle M, Craig E (2021) Overriding importance of water table in the greenhouse gas balance of managed peatlands. Nature 598:548–552
Fluet-Chouinard E, Stocker BD, Zhang Z, Malhotra A, Melton JR, Poulter B, Kaplan JO, Goldewijk KK, Siebert S, Minayeva T (2023) Extensive global wetland loss over the past three centuries. Nature 614:281–286
Gulati RD, van Donk E (2002) Lakes in the Netherlands, their origin, eutrophication and restoration: state-of-the-art review. Ecological Restoration of Aquatic and Semi-Aquatic Ecosystems in the Netherlands (NW Europe). Springer, Dordrecht, pp 73–106
Gupta G, Khan J, Upadhyay AK, Singh NK (2020) Wetland as a sustainable reservoir of ecosystem services: prospects of threat and conservation. Restoration of Wetland Ecosystem: A Trajectory towards a Sustainable Environment. Springer, Cham, pp 31–43
Higgs ES, Harris JA, Heger T, Hobbs RJ, Murphy SD, Suding KN (2018) Keep ecological restoration open and flexible. Nat Ecol Evol 2:580
Hobbs RJ, Arico S, Aronson J, Baron JS, Bridgewater P, Cramer VA, Epstein PR, Ewel JJ, Klink CA, Lugo AE (2006) Novel ecosystems: theoretical and management aspects of the new ecological world order. Glob Ecol Biogeogr 15:1–7
Hobbs RJ, Higgs E, Harris JA (2009) Novel ecosystems: implications for conservation and restoration. Trends Ecol Evol 24:599–605
Inglett KS, Inglett PW, Reddy KR (2011) Soil microbial community composition in a restored calcareous subtropical wetland. Soil Sci Soc Am J 75:1731–1740. https://doi.org/10.2136/sssaj2010.0424
IPCC (2021) Summary for Policymakers. In: Masson-Delmotte V, Zhai P, Pirani A, Connors SL, Péan C, Berger S, Caud N, Chen Y, Goldfarb L, Gomis MI, Huang M, Leitzell K, Lonnoy E, Matthews JBR, Maycock TK, Waterfield T, Yelekçi O, Yu R, Zhou B (eds) Climate Change 2021 The Physical Science Basis Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, pp 3–32
IPCC Report (2022) Climate change 2022: Impacts, Adaptation and Vulnerability. Summary for policymakers. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. United Nations Environment Programme. UNEP AR6, xxiii–xxxiii.
Jin H, van Leeuwen CHA, Temmink RJM, Bakker ES (2022) Impacts of shelter on the relative dominance of primary producers and trophic transfer efficiency in aquatic food webs: implications for shallow lake restoration. Freshw Biol 67:1107–1122. https://doi.org/10.1111/fwb.13904
Kayranli B, Scholz M, Mustafa A, Hedmark Å (2010) Carbon storage and fluxes within freshwater wetlands: a critical review. Wetlands 30:111–124. https://doi.org/10.1007/s13157-009-0003-4
Kentula ME (1996) Wetland restoration and creation. In: Fretwell JD, Williams JS, Redman PJ (eds) National Water Summary on Wetland Resources, USGS Water-Supply Paper 2425. U.S. Department of the Interior U.S. Geological Survey, Washington, pp 87–92
Langeveld CA, Segers R, Dirks BOM, van den Pol-van Dasselaar A, Velthof GL, Hensen A (1997) Emissions of CO2, CH4 and N2O from pasture on drained peat soils in the Netherlands. Eur J Agron 7:35–42
Meli P, Benayas JMR, Balvanera P, Ramos MM (2014) Restoration enhances wetland biodiversity and ecosystem service supply, but results are context-dependent: a meta-analysis. PLoS ONE. https://doi.org/10.1371/journal.pone.0093507
Morriën E, Hannula SE, Snoek LB, Helmsing NR, Zweers H, De Hollander M, Soto RL, Bouffaud ML, Buée M, Dimmers W, Duyts H, Geisen S, Girlanda M, Griffiths RI, Jørgensen HB, Jensen J, Plassart P, Redecker D, Schmelz RM, Schmidt O, Thomson BC, Tisserant E, Uroz S, Winding A, Bailey MJ, Bonkowski M, Faber JH, Martin F, Lemanceau P, De Boer W, Van Veen JA, Van Der Putten WH (2017) Soil networks become more connected and take up more carbon as nature restoration progresses. Nat Commun. https://doi.org/10.1038/ncomms14349
Noordhuis R (2014) Waterkwaliteit en ecologische veranderingen in het Markermeer-IJmeer. Landschap 1:13–22
Oertel C, Matschullat J, Zurba K, Zimmermann F, Erasmi S (2016) Greenhouse gas emissions from soils—A review. Chem Der Erde. https://doi.org/10.1016/j.chemer.2016.04.002
Oliveira Junior ES, Temmink RJM, Buhler BF, Souza RM, Resende N, Spanings T, Muniz CC, Lamers LPM, Kosten S (2019) Benthivorous fish bioturbation reduces methane emissions, but increases total greenhouse gas emissions. Freshw Biol 64:197–207. https://doi.org/10.1111/fwb.13209
Panchal P, Preece C, Peñuelas J, Giri J (2022) Soil carbon sequestration by root exudates. Trends Plant Sci 27:749–757. https://doi.org/10.1016/j.tplants.2022.04.009
Renault P, Ben-Sassi M, Bérard A (2013) Improving the MicroResp™ substrate-induced respiration method by a more complete description of CO2 behavior in closed incubation wells. Geoderma 207–208:82–91. https://doi.org/10.1016/j.geoderma.2013.05.010
Robroek BJM, Jassey VEJ, Kox MAR, Berendsen RL, Mills RTE, Cécillon L, Puissant J, Meima-Franke M, Bakker PAHM, Bodelier PLE (2015) Peatland vascular plant functional types affect methane dynamics by altering microbial community structure. J Ecol 103:925–934. https://doi.org/10.1111/1365-2745.12413
Saaltink R, Dekker SC, Griffioen J, Wassen MJ (2016) Wetland eco-engineering: measuring and modeling feedbacks of oxidation processes between plants and clay-rich material. Biogeosciences 13:4945–4957. https://doi.org/10.5194/bg-13-4945-2016
Saaltink R, Princen K, Wassen M (2019) De Marker Wadden als casus voor slibverwaarding. Vakbl Nat Bos Landsch 152:23–27
Sasikala S, Tanaka N, Wah Wah HSY, Jinadasa KBSN (2009) Effects of water level fluctuation on radial oxygen loss, root porosity, and nitrogen removal in subsurface vertical flow wetland mesocosms. Ecol Eng 35:410–417. https://doi.org/10.1016/j.ecoleng.2008.10.003
Schaufler G, Kitzler B, Schindlbacher A, Skiba U, Sutton MA, Zechmeister-Boltenstern S (2010) Greenhouse gas emissions from European soils under different land use: effects of soil moisture and temperature. Eur J Soil Sci 61:683–696. https://doi.org/10.1111/j.1365-2389.2010.01277.x
Sutton-Grier AE, Megonigal JP (2011) Plant species traits regulate methane production in freshwater wetland soils. Soil Biol Biochem 43:413–420. https://doi.org/10.1016/j.soilbio.2010.11.009
Tak V, Lexmond L, Robroek T (2023) Data from: water level and vegetation type control carbon fluxes in a newly-constructed soft-sediment wetland. https://doi.org/10.5281/zenodo.8123992
Temmink RJM, van den Akker M, Robroek BJM, Cruijsen PMJM, Veraart AJ, Kosten S, Peters RCJH, Verheggen-Kleinheerenbrink GM, Roelofs AW, van Eek X, Bakker ES, Lamers LPM (2021) Nature development in degraded landscapes: How pioneer bioturbators and water level control soil subsidence, nutrient chemistry and greenhouse gas emission. Pedobiologia 87–88:150745. https://doi.org/10.1016/j.pedobi.2021.150745
Temmink RJM, Lamers LPM, Angelini C, Bouma TJ, Fritz C, van de Koppel J, Lexmond R, Rietkerk M, Silliman BR, Joosten H, van der Heide T (2022a) Recovering wetland biogeomorphic feedbacks to restore the world’s biotic carbon hotspots. Science. https://doi.org/10.1126/science.abn1479
Temmink RJM, van den Akker M, van Leeuwen CHA, Thöle Y, Olff H, Reijers VC, Weideveld STJ, Robroek BJM, Lamers LPM, Bakker ES (2022b) Herbivore exclusion and active planting stimulate reed marsh development on a newly constructed archipelago. Ecol Eng 175:106474. https://doi.org/10.1016/j.ecoleng.2021.106474
Turner JC, Moorberg CJ, Wong A, Shea K, Waldrop MP, Turetsky MR, Neumann RB (2020) Getting to the Root of plant-mediated methane emissions and oxidation in a thermokarst bog. J Geophys Res Biogeosci. https://doi.org/10.1029/2020JG005825
Urakawa H, Bernhard AE (2017) Wetland management using microbial indicators. Ecol Eng 108:456–476. https://doi.org/10.1016/j.ecoleng.2017.07.022
van Bergen TJHM, Barros N, Mendonça R, Aben RCH, Althuizen IHJ, Huszar V, Lamers LPM, Lürling M, Roland F, Kosten S (2019) Seasonal and diel variation in greenhouse gas emissions from an urban pond and its major drivers. Limnol Oceanogr 64:2129–2139
van den Berg M, van den Elzen E, Ingwersen J, Kosten S, Lamers LPM, Streck T (2020) Contribution of plant-induced pressurized flow to CH4 emission from a Phragmites fen. Sci Rep 10:1–10. https://doi.org/10.1038/s41598-020-69034-7
van Leeuwen C, Temmink R, Jin H, Kahlert Y, Robroek BJM, Berg MP, Lamers LPM, den Akker M, Posthoorn R, Boosten A, Olff H, Bakker ES (2021) Enhancing ecological integrity while preserving ecosystem services: constructing soft-sediment islands in a shallow lake. Ecol Solut Evid 2:1–10. https://doi.org/10.1002/2688-8319.12098
van Leeuwen C, Temmink R, Jin H, Kahlert Y, Robroek BJM, Berg MP, Lammers L, van den Akker M, Posthoorn R, Boosten A, Olff H, Bakker E (2022) Ecosysteemherstel door vijf jaar oude Marker Wadden. Levende Nat 123:6
Verschoor M, Rijsdorp A (2012) Marker Wadden Sleutel voor een natuurrijk en toekomstbestendig Markermeer.
Vörösmarty CJ, Sahagian D (2000) Anthropogenic disturbance of the terrestrial water cycle. Bioscience 50:753–765
Vroom RJE, van den Berg M, Pangala SR, van der Scheer OE, Sorrell BK (2022) Physiological processes affecting methane transport by wetland vegetation-a review. Aquat Bot. https://doi.org/10.1016/j.aquabot.2022.103547
Whiting GJ, Chanton JP (1993) Primary production control of methane emission from wetlands. Nature 364:794–795. https://doi.org/10.1038/364794a0
Wiegleb G, Dahms H-U, Byeon WI, Choi G (2017) To what extent can constructed wetlands enhance biodiversity? Int J Environ Sci Dev 8:561–569. https://doi.org/10.18178/ijesd.2017.8.8.1016
Yang H, Tang J, Zhang C, Dai Y, Zhou C, Xu P, Perry DC, Chen X (2020) Enhanced carbon uptake and reduced methane emissions in a newly restored wetland. J Geophys Res Biogeosci 125:1–11. https://doi.org/10.1029/2019JG005222
Yu L, Huang Y, Sun F, Sun W (2017) A synthesis of soil carbon and nitrogen recovery after wetland restoration and creation in the United States. Sci Rep. https://doi.org/10.1038/s41598-017-08511-y
Acknowledgements
The authors would like to thank Roy Peters, Germa Verheggen, and Sebastian Krosse for their help with chemical analyses. We acknowledge the Gieskes-Strijbis Fund for financing the project and Natuurmonumenten for their facilitating our work on the Marker Wadden. BJMR is supported by the Dutch Science Foundation (grant no. ENW-M OCENW.M20.339).
Funding
This work was supported by Gieskes-Strijbis Fund and Nederlandse Organisatie voor Wetenschappelijk Onderzoek (Grant No. OCENW.M20.339)
Author information
Authors and Affiliations
Contributions
RJEV, BJMR and RJMT contributed to the design and implementation of the research. DT and RL performed the Microresp analyses. RJEV prepared all figures and performed the statistical analyses. DT, RJEV and RJMT wrote the main manuscript text, and all authors contributed to subsequent drafts.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tak, D.B.Y., Vroom, R.J.E., Lexmond, R. et al. Water level and vegetation type control carbon fluxes in a newly-constructed soft-sediment wetland. Wetlands Ecol Manage 31, 583–594 (2023). https://doi.org/10.1007/s11273-023-09936-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-023-09936-1