Abstract
Paddy fields in the agricultural landscape have become alternative habitats for natural wetland species. Habitat degradation, including habitat loss and fragmentation, is a major threat to members of Amphibia, which is a good indicator species for the Satoyama landscape. Recently, linear artificial structures, such as roads and railways, were recognized as factors inhibiting amphibian population persistence. Thus, irrigation canals and cement-walled streams may also affect amphibian movement and dispersal in the rural agricultural landscape termed Satoyama, which is now the main habitat for lowland wetland amphibians. However, there is limited information on such effects. Here, we focused on the Japanese brown frog (Rana japonica), which is an indicator species of the ecosystem health of the Satoyama landscape, to investigate the effects of irrigation canals on population structure. The barrier effects of roads and irrigation canals on gene flow between breeding sites were evaluated. We constructed a resistance map of the limiting factors to gene flow based on land use and frog movement and tested for correlations between cost and genetic distances. The habitat resistance values alone were insufficient to explain the genetic distances among breeding sites. Thus, we hypothesize that irrigation canals and roads have barrier effects on gene flow among amphibians in the Japanese Satoyama landscape.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wetland areas in lowlands are declining in size and experiencing strong development pressure (Malekmohammadi and Jahanishakib 2017). Globally, the wildlife, whose main habitat is lowland wetlands such as amphibians, are declining in population. On the other hand, lowland wetlands in the Asian region are often rice paddy fields, and many wetland animals and plants survive in paddy fields despite continuous human activity (Katoh and Ahern 2009; Kobori and Primack 2003; Lawler 2001). In these paddy fields as well as small natural wetlands in lowlands, grey-faced buzzard and herons are the top predators of the ecosystem. (Amira et al. 2018; Fujita et al. 2015; Katoh and Ahern 2009). Frogs are one of the main food resources for these birds and also predators of insects and soil animals; thus, egg masses of frogs have been monitored as indicators of ecosystem health in these paddy environments (Kuramoto and Sonoda 2003). However, even paddy fields in Asia have been degraded as habitats for frogs and other animals due to the modernization of agriculture, including drying out due to increase irrigation canals and paved roads, in recent years. (Hamer and McDonnell 2008; Kidera et al. 2018; Lane and Fujioka 1998; Tuomainen and Candolin 2011).
Japan is a region that has been modernized and developed from an early stage (about hundred years ago) in Asia; even now, the irrigation canals for paddy field management are increasing at a rate of 170 km per year. It has been noted that agricultural modernization and road maintenance have had substantial impacts on the wetland ecosystem (Natsuhara 2013). Paved roads also influence the gene flow of many amphibian species and other small animals, such as the wood frog (Lithobates sylvaticus), common frog (Rana temporaria), rattlesnakes (Crotalus horridus), and others (Buskirk 2012; Clark et al. 2010; Garcia-Gonzalez et al. 2012; Safner et al. 2011; Trochet et al. 2016). Linear barriers, such as highways and railways, both restrict migration and dispersal movements and also affect the population persistence of amphibians (Beebee 2013; Matos et al. 2019; Rytwinski and Fahrig 2012). In fact, it was reported that movement inhibition in the common brown frog (R. temporaria) occurs depending on the traffic volume of the road, which decreases gene flow between breeding ponds (Safner et al. 2011).
Paddy fields, which provide breeding environments for frogs in Asia, are spread over the lowlands, unlike the breeding ponds adjacent to forests that sporadically exist across suburban agricultural land. Therefore, linear structures such as irrigation canals and roads are thought to have different effects on frog breeding success. These linear structures, often present along with the paddy fields, may act as very strong barriers or as partitions between the breeding sites themselves. In paddy fields in Asia, linear structures can impede gene flow and the movement among forests and paddy fields, and cause habitat degradation as the paddy environment becomes fragmented. In Japan, it was reported that paddy field size affects the habitat potential for Japanese brown frogs (Natsuhara and Kanbara 2001). Therefore, there is a risk that roads and irrigation canals will narrow the breeding area by dividing paddy fields that appear uniform. Information on how irrigation canals and roads affect the health of local frog populations is very important for prioritizing impact mitigation measures for the conservation of wetland ecosystems in paddy fields. In our previous research, we revealed that roads and irrigation canals may have affected the gene flow between breeding areas of Japanese brown frogs in paddy fields, but the extent was not clarified (Kobayashi et al. 2013; Kobayashi and Abe 2019). The Japanese brown frog has no suckers, and adults inhibit forest edges and in forests, making it more susceptible to concrete structures and development than other frogs that breed in paddy fields (Watabe et al 2010). If the health of local populations of Japanese brown frog is compromised by disruption of migration and gene flow, frog populations will decline and the conservation of wildlife at the top of the wetland ecosystem that feed on frogs, such as gray-faced buzzards and white storks that are considered for reintroduction, will be affected. Thus, the health of the Japanese brown frog population is one important indicator of the balance and health of wetland ecosystems.
Therefore, in this study, we estimated to what extent irrigation canals and roads affect the genetic diversity of the Japanese brown frog. Specifically, the purpose of this study was to clarify the degree of gene flow inhibition of roads and irrigation canals that separated breeding sites of Japanese brown frog, using landscape genetic analysis.
Material and methods
Study area
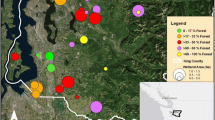
The study was conducted in a 3-km2 area on the west side of Inba Lake, Chiba Prefecture, Japan. The study area contains a two-way, 10-m wide road and a 3-m wide, cement-walled irrigation canal, named the Moroto River (Fig. 1). The surrounding area is characterized by various land uses (Fig. 1). To the east of the study area, there is a lake, a few busy roads, and an urban area. The north side of the study area consists of a golf course, a large railway, paddy fields, and an urban area with a large road. To the west of the study area, there is a large golf course. To the south of the study area, there is a paddy field and a lake.
Land cover map
We constructed a land cover map based on the National Actual Vegetation Map of Japan (Fig. 1). Recently, new construction has expanded in this region. Thus, we revised a land cover map using data from a multispectral satellite image from GeoEye-1 (JAPAN SPACE IMAGING Co., Tokyo, Japan) taken in April 2009 (NIR/Red/Green/Blue, Pixel size = 1.64 m) and a ground survey conducted in 2012. We converted the polygon vector of the land cover map to a raster cell size of 1 m, which is appropriate for calculating least-cost distances because it avoids the cracks between the cost cells (Watts et al. 2016).
Definition of the study-species habitat for resistance values
We reviewed a previous study that described the suitable habitat for R. japonica to determine the resistance value of each land use. Those studies revealed that the primary habitats of this species are grasslands, paddy fields, and wetlands (Kaneko and Matsui 2004; Matsushima and Kawata 2005), and forest floors and the forest edge (Osawa and Katsuno 2001, 2002). Semi-habitats are defined as areas that are likely not optimal but may still provide resources for some life stages (Johansson et al. 2005; Safner et al. 2011); this includes crop fields in this area for R. japonica (Natsuhara and Kanbara 2001; Osawa and Katsuno 1997). Areas of unsuitable habitat (non-habitat) include artificial land uses, such as factories, residential areas, and commercial stores.
Resistance values and cost distances
We reviewed the resistance values that were defined for primary habitat, semi-habitat, non-habitat, and barriers in previous studies of the genus Rana (including Lithobates) since 2009 (Charney 2012; Coster et al. 2015; Decout et al. 2012; Gabrielsen et al. 2013; Patrick et al. 2012; Peterman et al. 2013; Pontoppidan and Nachman 2013; Richardson 2012; Safner et al. 2011; Scherer et al. 2012; Zellmer and Knowles 2009; Table 1). The ranges of values were 1 to 10 for primary habitat, 2 to 50 for semi-habitat, 5 to 220 for non-habitat, and 2 to infinity, which is indicative of being impassable, for barriers (Table 1). The values for each category varied because of the specific aims of each study. The values for semi-habitat tended to be 2 to 10 times those of primary habitats, whereas the values for non-habitats were 5 to 80 times greater than those for primary habitats. In this study, we used the median values at the smaller end of the range of previous studies as resistance values. The resistance values were as follows: primary habitat = 1, semi-habitat = 5, and non-habitat = 40 (Table 2).
We tested six combinations of barrier values (Table 2). First, we tested a “No Barrier” condition (case 1) to determine whether land use alone sufficiently explained the genetic distances. For the additional cases, we added high- (1000) and mid-resistance (200) values for barriers (roads and irrigation canals) to determine whether either or both affected genetic distances (Table 2, cases 2–6). We calculated cost distances among the 13 breeding sites using a function of the least cost path in ArcGIS toolbox ver. 9.3 (ESRI, Redlands, CA, USA) and correlation coefficients with genetic distances.
Field sampling
We conducted annual censuses counting the number of R. japonica egg masses in the study area from 2010 to 2012 (Fig. 1, Table 3). Many R. japonica individuals congregate to mate in wet paddy fields, ponds, and wetlands during the reproductive season, which is typically from February to March (Kaneko and Matsui 2004). From 2010 to 2012, we collected R. japonica eggs from 13 breeding sites within the study area (Fig. 1, Table 3) from February to March. The breeding sites were primarily in paddy fields, small drains beside paddy fields, and wetlands resulting from abandoned paddy fields. We collected approximately three eggs per egg mass from 20 egg masses each year at each breeding site (except site 1 in 2011). The latitude and longitude of each breeding site, and the numbers of samples and observed egg masses for each year are presented in Table 3.
DNA extraction
We reared the eggs to the tail-bud stage at room temperature (about 20 °C) in the laboratory. A few samples per breeding site did not reach the tail-bud stage and were, therefore, excluded from subsequent analyses. One individual per egg mass was euthanized for DNA extraction.
Total DNA was extracted using a DNeasy Blood and Tissue Kit (Qiagen Inc., Valencia, CA, USA). We used six loci (Raja01, -02, -03, -04, -10, and -19 from Koizumi et al. 2009) at which a bias for null alleles was not detected using Micro-checker 2.2.3 (Van Oosterhout et al. 2004). These markers were amplified by two multiplex PCR assays and one single-locus PCR assay as follows: multiplex-1 included Raja01, -03, and -04; multiplex-2 included Raja02 and Raja10; and the single-locus was Raja19. We carried out PCR for the different loci using the same PCR conditions, as follows: template DNA was added to 10 ml of PCR mixture consisting of 20 mM Tris–HCl (pH 8.0), 100 mM KCl, 20 mM MgCl2, dNTPs (2.5 mM each), 1 mg bovine serum albumin, 4% dimethyl sulfoxide, primers (20 mM each), and 0.5 U Takara Ex Taq DNA Polymerase Hot-Start Version (Takara Bio., Shiga, Japan). The PCR consisted of an initial denaturation step of 98 °C for 1 min, followed by 35 cycles of denaturation at 98 °C for 10 s, annealing at 60 °C for 60 s, and extension at 72 °C for 30 s, followed by a final extension at 60 °C for 30 min. Amplified fragments of microsatellite loci were genotyped using an ABI PRISM 3130xl Genetic Analyzer and GeneMapper 4.0 software (Thermo Fisher Scientific Inc., Waltham, MA, USA). Raja19 samples were mixed with multiplex-1 samples when loaded into capillary tubes. These egg samples and genetic data sets were used in our previous study (Kobayashi et al. 2018).
Genetic distances
The degree of genetic differentiation between populations is often measured by the fixation index GST (Nei 1973). G″ST is a standardized GST that corrects for the bias caused by a small number of populations. We calculated the pairwise G″ST values (Hedrick 2005; Meirmans and Hedrick 2011) to represent genetic distances using GenALEx 6.5.01 (Peakall and Smouse 2012) and genotyped data.
Simple and partial Mantel tests
We conducted simple and partial Mantel (P Mantel) tests to determine the correlations between genetic and cost distances using the Ecodist package in R 3.0.0 (Goslee and Urban 2007). We removed the effects of simple geographic distances on the P Mantel tests.
Additionally, to estimate the geographic range of random mating in this area, we calculated the correlation coefficient (Mantel r) for every 300-m geographic distance class using the Ecodist package and constructed a correlogram plot. The 300-m distance resulted from dividing the maximum geographic distance (~ 3000 m) into 10 classes.
Results
The geographic, genetic, and cost distances for the six cases are shown in Table 5. The maximum geographic distance was 2 996.6 m between sites No. 3 and No. 10, and the maximum G″ST value was 0.202 between No. 6 and No. 9. The minimum geographic distance was 232.9 m between sites No. 4 and No. 5, and the minimum G″ST value was 0.009 also between No. 4 and No. 5 (Table 5). There were significant correlations between the genetic and cost distances for all the cases we tested. The values of the correlation coefficients of both Mantel tests (Mantel r and P Mantel r) are shown in Table 4. The lowest correlation coefficient (Mantel r) occurred with Case 3, the “Only Canal” case, and the second lowest Mantel r value occurred with Case 1, the “No Barrier” case. On the contrary, the highest Mantel r value occurred with Case 5, the “Road > Canal” case, and the second highest Mantel r occurred with Case 4, the “Road = Canal” case.
The P Mantel tests, which determine the correlations between genetic and cost distances controlled by geographic distance, revealed that only three cases were significant. The highest correlation coefficient (P Mantel r) occurred with Case 5, the “Road > Canal” case, and the second highest P Mantel r occurred with Case 4, the “Road = Canal” case.
In Fig. 2, the correlation coefficient for each geographic class is shown in the correlogram. The correlation coefficients were high for the 450-m class but declined thereafter, reaching 0 for the 1000-m class. The Mantel tests of each class yielded significant correlations for the 150-, 450- (positive correlation) and 2850-m (negative correlation) classes only.
Correlogram plot of the genetic correlation coefficient (r) as a function of distance for the Japanese brown frog (Rana japonica). The extent of the positive spatial genetic structure as measured by the intercept was 300 m. The null hypothesis of a random distribution of genotypes is bound by 95% confidence intervals with error bars determined by bootstrapping. Dark circles represent significant values of the Mantel tests (p < 0.05). *Value exceeds the Bonferroni-corrected significance level (α/k = 0.05/10 = 0.005, where k is the number of distance classes)
Discussion
Our results suggested that genetic distances between R. japonica samples at different breeding sites could not be explained by the fragmentation of suitable habitat alone because the correlation coefficient of the “No Barrier” case was among the lowest of the tested cases. The highest and second highest correlation coefficients occurred with the “Road > Canal” and “Road = Canal” cases, respectively, in both the Mantel and P Mantel tests. Thus, both the roads and canals act as barriers to gene flow, although the roads have stronger barrier effects.
Previous studies indicated that the barrier effects of roads on migration and gene flow depended on the traffic volume and road width (Decout et al. 2012; Gabrielsen et al. 2013; Safner et al. 2011). The roads in our study area were ~ 10-m wide, with two-way traffic of ~ 700 vehicles/h (road traffic census data, Chiba Prefecture, Japan, 2010). These are mid-sized roads compared with larger roads, such as highways that were involved in previous studies, and those authors used the same resistance value as for a non-habitat area (Charney 2012; Coster et al. 2015; Decout et al. 2012; Safner et al. 2011). However, our results suggest that the mid-sized roads have a higher resistance value than non-habitat for this frog. Safner et al. (2011) used a threshold for impassibility of 1000 vehicles/h on a two-way street, which exceeds the traffic volume in our study. We presumed that the traffic volume threshold for impassability varies relative to the body size of adult animals. The body size of an adult R. japonica (3.5–6.5 cm) (Marunouchi et al. 2002) is smaller than that of R. temporaria (5.0–8.6 cm) (Miaud et al. 1999). Thus, the smaller streets in our study area may act as barriers to migration and restrict the gene flow of R. japonica.
Additionally, large rivers act as barriers to migration for some frog species (Angelone et al. 2011; Coster et al. 2015). However, smaller rivers, such as first- or second-order streams (upstream), may either form a barrier to migration or be suitable habitat, depending on the species (Charney 2012; Coster et al. 2015; Decout et al. 2012). Compared with a previous study, the resistance value (200) in our study corresponded to the barrier effects of a third- or fourth-order river (downstream) on R. sylvatica (Coster et al. 2015). The under-developed suckers on the hands and feet of R. japonica make it difficult for them to climb cement walls (Watabe et al. 2012). Therefore, the irrigation canal (3-m width) within this study area, which is the size of a second-order stream, formed a barrier with the effect of a large river to migration and gene flow. The resistance value of a canal is high because of the cement walls.
In our study, the resistance value of roads was higher than that of irrigation canals. This may result from the crossability. Frogs may be able to cross the canal using riparian and floating vegetation by adhering to the cement wall or using narrow pedestrian bridges. However, it may be more difficult for frogs to cross the paved, hot, and dry roads while avoiding vehicles.
However, even in this fine-scale landscape, the Mantel test-based correlogram revealed the effects of isolation owing to distance (Fig. 2). The effective dispersal range of this species appears to be between 500 and 1000 m within the study area (Fig. 2). This is consistent with our previous study in this area, in which we estimated the dispersal range to be ~ 600 m using assignment test-based estimations of the breeding sites of each captured individual (Kobayashi and Abe 2019). Additionally, the range is consistent with findings of other studies on R. japonica, in which the home range has been estimated as 200 to 270 m, with a maximum of 500 m (Osawa and Katsuno 2001), and with studies on other ranid frog species (500 to 1000 m; Berven and Grudzien 1990; Dole 1971). Long-distance dispersal (e.g., over 5 km) has been documented in rare instances for some ranid frogs, such as R. pipiens and R. clamitans (Berven and Grudzien 1990; Dole 1971; Schroeder 1976). However, this capability for R. japonica in the study area is hindered by the presence of linear barriers.
This study confirmed that roads and concrete-walled canals act as barriers to the movement of Japanese brown frogs, which may also occur in other small animals such as other amphibians and mammals. When considering the health of wetland ecosystems, our results suggest that road conservation measures are a priority. Because amphibian roadkill directly affects populations, conservation measures have been studied (Lesbarrères et al. 2004; Taylor and Goldingay 2010; Wang et al. 2019). In Asia, the effectiveness of underpasses has been confirmed for brown frogs and toads, and it is thought that multiple lined-with-soil underpasses are particularly effective for roads that cross paddy fields or wetlands (Wang et al. 2019). Previous studies have also shown that concrete walls and ditches act as barriers in ranid frogs such as Japanese pond frogs (Pelophylax porosua porosua) that do not have suction cups and sufficient jumping capabilities to cross over (Watabe et al. 2010, 2011). In previous research, conservation measures such as attaching slopes and nets and making the surface uneven have been examined and their effects have been confirmed (Watabe et al. 2010, 2011). In paddy field areas near urban areas where suitable habitats are decreasing because of paddy improvements and abandonment, it is necessary to actively adopt such conservation measures to balance biodiversity conservation and urban functions in wetland ecosystems.
References
Amira N, Rinalfi T, Azhar B (2018) Effects of intensive rice production practices on avian biodiversity in Southeast Asian managed wetlands. Wet Ecol Manag 26:865–877. https://doi.org/10.1007/s11273-018-9614-y
Angelone S, Kienast F, Holderegger R (2011) Where movement happens: scale-dependent landscape effects on genetic differentiation in the European tree frog. Ecography 34:714–722. https://doi.org/10.1111/j.1600-0587.2010.06494.x
Beebee TJC (2013) Effects of road mortality and mitigation measures on amphibian populations. Conserv Biol 27:657–668. https://doi.org/10.1111/cobi.12063
Berven KA, Grudzien TA (1990) Dispersal in the wood frog (Rana sylvatica): implications for genetic population structure. Evolution 44:2047–2056. https://doi.org/10.2307/2409614
Buskirk J (2012) Permeability of the landscape matrix between amphibian breeding sites. Ecol Evol 2:3160–3167. https://doi.org/10.1002/ece3.424
Charney ND (2012) Evaluating expert opinion and spatial scale in an amphibian model. Ecol Modell 242:37–45. https://doi.org/10.1016/j.ecolmodel.2012.05.026
Clark RW, Brown WS, Stechert R, Zamudio KR (2010) Roads, interrupted dispersal, and genetic diversity in timber rattlesnakes. Conserv Biol 24:1059–1069. https://doi.org/10.1111/j.1523-1739.2009.01439.x
Coster SS, Babbitt KJ, Kovach AI (2015) High genetic connectivity in wood frogs (Lithobates sylvaticus) and spotted salamanders (Ambystoma maculatum) in a commercial forest. Herpetol Conserv Biol 10:64–89
Decout S, Manel S, Miaud C, Luque S (2012) Integrative approach for landscape-based graph connectivity analysis: a case study with the common frog (Rana temporaria) in human-dominated landscapes. Landsc Ecol 27:267–279. https://doi.org/10.1007/s10980-011-9694-z
Dole JW (1971) Dispersal of recently metamorphosed Leopard Frogs, Rana pipiens. Copeia 1971:221–228. https://doi.org/10.2307/1442821
Fujita G, Naoe S, Miyashita T (2015) Modernization of drainage systems decreases gray-faced buzzard occurrence by reducing frog densities in paddy-dominated landscapes. Landsc Ecol Eng 11:189–198. https://doi.org/10.1007/s11355-014-0263-x
Gabrielsen CG, Kovach AI, Babbitt KJ, McDowell WH (2013) Limited effects of suburbanization on the genetic structure of an abundant vernal pool-breeding amphibian. Conserv Genet 14:1083–1097. https://doi.org/10.1007/s10592-013-0497-4
Garcia-Gonzalez C, Campo D, Pola IG, Garcia-Vazquez E (2012) Rural road networks as barriers to gene flow for amphibians: species-dependent mitigation by traffic calming. Landsc Urban Plan 104:171–180. https://doi.org/10.1016/j.landurbplan.2011.10.012
Goslee SC, Urban DL (2007) The ecodist package for dissimilarity-based analysis of ecological data. J Stat Softw 22:1–19
Hamer AJ, McDonnell MJ (2008) Amphibian ecology and conservation in the urbanising world: a review. Biol Conserv 141:2432–2449. https://doi.org/10.1016/j.biocon.2008.07.020
Hedrick PW (2005) A standardized genetic differentiation measure. Evolution 59:1633–1638
Johansson M, Primmer CR, Sahlsten J, Merila J, Centre EB, Johansson M, Primmer CR, Sahlsten J, Merila J, Merilä J (2005) The influence of landscape structure on occurrence, abundance and genetic diversity of the common frog, Rana temporaria. Glob Change Biol 11:1664–1679. https://doi.org/10.1111/j.1365-2486.2005.01005.x
Kaneko Y, Matsui M (2004) Rana japonica. In: Bailie JEM, Hilton-Taylor C, Stuart SN (eds) 2004 IUCN red list of threatened species: a global species assessment. IUCN, Gland. https://doi.org/10.2305/IUCN.UK.2004.RLTS.T58625A11815180.en
Katoh S, Ahern J (2009) Multifunctional landscapes as a basis for sustainable landscape development. J Jpn Inst Landsc Archit 72:799–804. https://doi.org/10.5632/jila.72.799
Kidera N, Kadoya T, Yamano H, Takamura N, Ogano D, Wakabayashi T, Takezawa M, Hasegawa M (2018) Hydrological effects of paddy improvement and abandonment on amphibian populations; long-term trends of the Japanese brown frog, Rana japonica. Biol Conserv 219:96–104. https://doi.org/10.1016/j.biocon.2018.01.007
Kobayashi S, Abe S (2019) Estimated dispersal range of Japanese brown frogs using assignment tests. Bull Herpetol Soc Jpn 2:127–136 (in Japanese)
Kobayashi S, Abe S, Matsuki R (2013) Genetic structure of a Japanese brown frog (Rana japonica) population implies severe restriction of gene flow caused by recent urbanization in a satoyama landscape. Mitochondrial DNA 24:697–704. https://doi.org/10.3109/19401736.2013.773981
Kobayashi S, Abe S, Tomita M, Matsuki R (2018) Fine-scale genetic structure and estimation of gene flow of the Japanese brown frog Rana japonica in a satoyama landscape on the western side of Inba Lake, Eastern Japan. Curr Herpetol 37:11–22. https://doi.org/10.5358/hsj.37.11
Kobori H, Primack RB (2003) Participatory conservation approaches for satoyama, the traditional forest and agricultural landscape of Japan. Ambio 32:307–311
Koizumi N, Watabe K, Mori A, Takemura T (2009) Isolation and characterization of 19 polymorphic microsatellite DNA markers in the Japanese brown frog (Rana japonica). Mol Ecol Res 9:248–250. https://doi.org/10.1111/j.1755-0998.2008.02429.x
Kuramoto N, Sonoda Y (2003) Biological diversity in satoyama landscapes. In: Takeuchi K, Brown RD, Washitani I, Tsunekawa A, Yokohari M (eds) satoyama. Springer, Tokyo, pp 81–109. https://doi.org/10.1007/978-4-431-67861-8_4
Lane SJ, Fujioka M (1998) The impact of changes in irrigation practices on the distribution of foraging egrets and herons (Ardeidae) in the rice fields of central Jpn. Biol Conserv 83:221–230. https://doi.org/10.1016/S0006-3207(97)00054-2
Lawler P (2001) Rice fields as temporary wetlands: a review. Israel J Zool 47:513–528
Lesbarrères D, Lodé T, Merilä J (2004) What type of amphibian tunnel could reduce road kills? Oryx 38:220–223. https://doi.org/10.1017/S0030605304000389
Malekmohammadi B, Jahanishakib F (2017) Vulnerability assessment of wetland landscape ecosystem services using driver-pressure-state-impact-response (DPSIR) model. Ecol Indic 82:293–303. https://doi.org/10.1016/j.ecolind.2017.06.060
Marunouchi J, Kusano T, Ueda H (2002) Fluctuation in abundance and age structure of a breeding population of the Japanese brown frog, Rana japonica Gunther (Amphibia, Anura). Zool Sci 19:343–350. https://doi.org/10.2108/zsj.19.343
Matos C, Petrovan SO, Wheeler PM, Ward AI (2019) Landscape connectivity and spatial prioritization in an urbanising world: a network analysis approach for a threatened amphibian. Biol Conserv 237:238–247. https://doi.org/10.1016/j.biocon.2019.06.035
Matsushima N, Kawata M (2005) The choice of oviposition site and the effects of density and oviposition timing on survivorship in Rana japonica. Ecol Res 20:81–86. https://doi.org/10.1007/s11284-004-0010-0
Meirmans PG, Hedrick PW (2011) Assessing population structure: FST and related measures. Mol Ecol Resour 11:5–18. https://doi.org/10.1111/j.1755-0998.2010.02927.x
Miaud C, Guyétant R, Elmberg J (1999) Variations in life-history traits in the common frog Rana temporaria (Amphibia: Anura): a literature review and new data from the French Alps. J Zool 249:61–73. https://doi.org/10.1017/S0952836999009061
Natsuhara Y (2013) Ecosystem services by paddy fields as substitutes of natural wetlands in Japan. Ecol Eng 56:97–106. https://doi.org/10.1016/j.ecoleng.2012.04.026
Natsuhara Y, Kanbara M (2001) Habitat suitability and network for the Japanese brown frog, Rana japonica Gunther in Southern Osaka. Jpn Inst Landsc Archit 64:617–620. https://doi.org/10.5632/jila.64.617
Nei M (1973) Analysis of gene diversity in subdivided populations. PNAS USA 70:3321–3323
Osawa S, Katsuno T (1997) Discussion of peculiarity in Yato (paddy field at bottomland with sideslope) and conservation of frogs. J Jpn Inst Landsc Archit 61:529–534. https://doi.org/10.5632/jila.61.529
Osawa S, Katsuno T (2001) Dispersal of brown frogs Rana japonica and R. ornativentris in the forests of the Tama Hills. Curr Herpetol 20:1–10. https://doi.org/10.5358/hsj.20.1
Osawa S, Katsuno T (2002) Environmental factors influencing the relative abundance of brown frogs in urban area ecological parks. J Jpn Inst Landsc Archit 65:513–516. https://doi.org/10.5632/jila.65.513
Patrick DA, Gibbs JP, Popescu VD, Nelson DA (2012) Multi-scale habitat-resistance models for predicting road mortality “hotspots” for turtles and amphibians. Herpetol Conserv Biol 7:407–426
Peakall R, Smouse PE (2012) GenALEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 28:2537–2539. https://doi.org/10.1093/bioinformatics/bts460
Peterman WE, Feist SM, Semlitsch RD, Eggert LS (2013) Conservation and management of peripheral populations: spatial and temporal influences on the genetic structure of wood frog (Rana sylvatica) populations. Biol Conserv 158:351–358. https://doi.org/10.1016/j.biocon.2012.07.028
Pontoppidan M-B, Nachman G (2013) Spatial Amphibian impact assessment—a management tool for assessment of road effects on regional populations of Moor frogs (Rana arvalis). Nat Conserv 5:29–52. https://doi.org/10.3897/natureconservation.5.4612
Richardson JL (2012) Divergent landscape effects on population connectivity in two co-occurring amphibian species. Mol Ecol 21:4437–4451. https://doi.org/10.1111/j.1365-294X.2012.05708.x
Rytwinski T, Fahrig L (2012) Do species life history traits explain population responses to roads? A meta-analysis. Biol Conserv 147:87–98. https://doi.org/10.1016/j.biocon.2011.11.023
Safner T, Miaud C, Gaggiotti O, Decout S, Rioux D, Zundel S, Manel S (2011) Combining demography and genetic analysis to assess the population structure of an amphibian in a human-dominated landscape. Conserv Genet 12:161–173. https://doi.org/10.1007/s10592-010-0129-1
Scherer RD, Muths E, Noon BR (2012) The importance of local and landscape-scale processes to the occupancy of wetlands by pond-breeding amphibians. Popul Ecol 54:487–498. https://doi.org/10.1007/s10144-012-0324-7
Schroeder EE (1976) Dispersal and movement of newly transformed green frogs, Rana clamitans. Am Midl Nat 95:471–474. https://doi.org/10.2307/2424413
Taylor BD, Goldingay RL (2010) Roads and wildlife: impacts, mitigation and implications for wildlife management in Australia. Wildl Res 37:320–331
Trochet A, Dechartre J, Chevalier HL, Baillat B, Calvez O, Blanchet S, Ribéron A (2016) Effects of habitat and fragmented-landscape parameters on amphibian distribution at a large spatial scale. Herpetol J 26:73–73
Tuomainen U, Candolin U (2011) Behavioural responses to human-induced environmental change. Biol Rev 86:640–657. https://doi.org/10.1111/j.1469-185X.2010.00164.x
Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538. https://doi.org/10.1111/j.1471-8286.2004.00684.x
Wang Y, Lan J, Zhou H, Guan L, Wang Y, Han Y, Qu J, Shah SA, Kong Y (2019) Investigating the effectiveness of road-related mitigation measures under semi-controlled conditions: a case study on Asian amphibians. Asian Herpetol Res 10:62–68
Watabe K, Mori A, Koizumi N, Takemura T (2010) Fundamental experiments to develop eco-friendly techniques for conserving frog habitat in paddy areas: escape countermeasures for frogs falling into agricultural concrete canals. Jpn Agric Res Q 44:405–413. https://doi.org/10.6090/jarq.44.405
Watabe K, Mori A, Koizumi N, Takemura T, Park MS (2011) Experiment for development of simple escape countermeasures for frogs falling into concrete canals. Trans Jpn Soc Irrig Drain Rural Eng 79:215–221. https://doi.org/10.11408/jsidre.79.215
Watabe K, Senga Y, Mori A, Koizumi N, Takemura T, Nishida K (2012) Population model of Rana japonica crossing agricultural concrete canals: evaluating population conservation by improving the migration routes of frogs. Paddy Water Environ 10:223–229. https://doi.org/10.1007/s10333-012-0313-7
Watts K, Vanhala T, Connolly T, Cottrell J (2016) Striking the right balance between site and landscape-scale conservation actions for a woodland insect within a highly fragmented landscape: a landscape genetics perspective. Biol Conserv 195:146–155. https://doi.org/10.1016/j.biocon.2015.12.039
Zellmer AJ, Knowles LL (2009) Disentangling the effects of historic vs. contemporary landscape structure on population genetic divergence. Mol Ecol 18:3593–3602. https://doi.org/10.1111/j.1365-294X.2009.04305.x
Acknowledgements
We thank Ms. Ishiyama and Mr. Furukawa (CRECE Inc.) for their support with sampling and their technical assistance. We thank Lesley Benyon, PhD, and Mallory Eckstut, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Funding
This research was carried out using in-house funding. Therefore, neither author will receive any direct financial benefits from publication.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by SK and SA. The first draft of the manuscript was written by SK and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1
Appendix 1
See Table 5.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kobayashi, S., Abe, S. The effects of irrigation canals and roads as barriers to gene flow between Japanese brown frog (Rana japonica) breeding sites at a fine scale in a Satoyama landscape. Wetlands Ecol Manage 31, 191–202 (2023). https://doi.org/10.1007/s11273-023-09910-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-023-09910-x