Abstract
Wetlands are important global carbon sinks, an increasingly important ecosystem service. Invasive plants can disrupt wetland carbon budgets, although efforts to suppress invasive plants may also have unintended effects. Invasive Phragmites australis subsp. australis (European common reed) produces extensive monocultures that displace resident plant communities. In Long Point (Ontario, Canada), a glyphosate-based herbicide was used to control over 900 ha of P. australis. We determined how this ongoing management, and different environmental conditions, influence net primary productivity and decomposition rates. We compared above- and below-ground biomass, belowground:aboveground biomass ratios, standing dead stems, and litter in un-treated P. australis stands, in herbicide-treated areas, and in reference vegetation. We also conducted a reciprocal transplant experiment to measure the decomposition rates of plant litter. One-year post-treatment, control efforts dramatically reduced aboveground biomass (122 g/m2 ± 133 SD) compared to un-treated P. australis (1254 g/m2 ± 449 SD) and reference habitat (821 g/m2 ± 335 SD). An interaction between the plant community of the site and water depth predicted litter decomposition rates, with litter loss ranging from 5.75 to 74.65% across all species, and submerged litter decomposing faster. These results emphasize that secondary treatment, such as rolling, burning, or cutting, encourages the decomposition of dead P. australis stems, opening up space for the recovery of native plants. While herbicide-treated sites had low biomass immediately following treatment, aquatic vegetation began rapidly colonizing treated areas, suggesting that plant community recovery may restore the wetland carbon uptake in subsequent years.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Wetlands are global sinks of carbon (Mitsch et al. 2013). While vegetation plays a critical role in carbon assimilation (Rothman and Bouchard 2007; Schultz et al. 2011), the spread of an invasive plant can disrupt carbon budgets. Invasive plants can influence carbon cycling directly by having higher primary production than native species (e.g., Lei et al. 2019) or producing more nutrient-rich litter (e.g., Yuckin and Rooney 2019), or indirectly, by changing environmental factors such as light penetration, oxygen availability, or water depths (e.g., Robichaud and Rooney 2021a). Broadly, plant invasions tend to increase the size of the carbon pool and the rate of carbon cycling in wetlands (Liao et al. 2008; Vilà et al. 2011). However, the impact of any individual invasive species will depend on the properties of the invader, the invaded system, and the displaced plant community (Ehrenfeld 2003, 2010; Liao et al. 2008). Thus, the ecological consequences of invasion are system-specific.
Invasive Phragmites australis ssp. australis (hereafter P. australis) is a problematic invader in North American wetlands (Chambers et al. 1999). A perennial grass, P. australis has high carbon assimilation rates (Farnsworth and Meyerson 2003; Tho et al. 2016; Robichaud and Rooney 2021b) and produces considerable amounts of above- and below-ground biomass (Windham 2001; Rothman and Bouchard 2007; Duke et al. 2015; Lei et al. 2019) that create dense stands. These stands become more dense with age as dead stems remain upright and accumulate for 3 or more years (Rooney lab, unpublished data). The productivity of P. australis coupled with the recalcitrant nature of its stem litter suggests that P. australis invasion may increase carbon sequestration and storage in some ecosystems. However, P. australis has relatively high foliar nitrogen concentrations (Findlay et al. 2002; Hirtreiter and Potts 2012; Yuckin and Rooney 2019), which could expedite decomposition. Thus, the direct influence of P. australis invasion on the carbon budget is unclear.
Phragmites australis invasion is also likely to influence carbon cycling indirectly. In some wetland habitats, P. australis can reduce the depths of standing water (Windham and Lathrop 1999) and intercept more light than native plant communities (Hirtreiter and Potts 2012). Such alterations to environmental conditions can drive changes in primary production and decomposition rates. Deeper standing water can increase macrophyte biomass and decomposition rates (van der Valk et al. 1991; Christensen et al. 2009; Duke et al. 2015), while greater light interception creates shading that can reduce water temperatures, which mediate the rate of decomposition (Hirtreiter and Potts 2012).
Due to the documented negative effects of P. australis invasion on wetland biota (e.g., Greenberg and Green 2013; Robichaud and Rooney 2017), there have been extensive efforts to control P. australis in North America (Martin and Blossey 2013; Hazelton et al. 2014). Most of these control projects apply herbicide, such as glyphosate or imazapyr, and then follow this approach with a secondary treatment such as rolling or mowing to remove standing dead biomass (e.g., Hazelton et al. 2014). Controlling an established invasive species is challenging, and treatment often requires the evaluation of potential trade-offs between habitat conservation and other ecosystem services (e.g., Alldred et al. 2016). Reducing P. australis populations in a marsh is likely to improve habitat quality for wildlife (e.g., Schummer et al. 2012; Markle and Chow-Fraser 2018), but this may be at the cost of reduced carbon sequestration—an essential ecosystem service (IPCC 2021).
Working alongside an ongoing P. australis suppression project, we measured changes in primary production and decomposition to provide insight into the effects that invasion and herbicide treatment have on short-term carbon sequestration in freshwater marshes. Our objectives were to (1) quantify the effect of invasive P. australis on freshwater coastal marsh carbon dynamics and relevant environmental conditions and (2) evaluate if, in the short-term, herbicide-based P. australis suppression restores primary production and decomposition to rates observed in equivalent uninvaded ‘reference’ marsh. We predicted that primary production would decrease and decomposition would increase in herbicide-treated sites compared to both un-treated P. australis and reference plant communities because of changes to the plant community, litter composition, and environmental conditions.
Methods
Study location
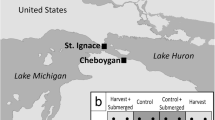
Our study took place in Long Point (42° 34′ N, 80° 24′ W), a peninsula located on the north shore of Lake Erie, Ontario, Canada (Fig. 1). In Long Point, invasive P. australis populations have been growing since the late 1990s, and most frequently replaced meadow marsh and cattail marsh plant communities (Wilcox et al. 2003). To contend with P. australis invasion, the Ontario Ministry of Northern Development, Mines, Natural Resources and Forestry (NDMNRF) began a P. australis control project in these marshes. In 2016, the NDMNRF obtained an Emergency Use Registration (no. 32356) under the Pest Control Products Act from Health Canada’s Pest Management Regulation Authority and a provincial Permit to Perform an Aquatic Extermination of invasive P. australis to apply a glyphosate-based herbicide [Roundup® Custom for Aquatic & Terrestrial Use Liquid Herbicide (Bayer CropScience, Whippany, New Jersey, USA)] combined with a nonionic alcohol ethoxylate surfactant (Aquasurf®, registration no. 32152, Brandt Consolidated, Springfield, IL, USA), to control P. australis (OMNRF 2016). This custom herbicide blend was formulated specifically to be safe for over-water use, and concentrations in the water and sediment never reached thresholds of concern for aquatic biota (Robichaud and Rooney 2021c). In September 2016, herbicide was applied to approximately 355 ha across the Long Point peninsula at a rate of 4210 g acid equivalent (a.e.) glyphosate/ha as an isopropylamine salt combined with 0.5 L/ha of Aquasurf® via helicopter (Eurocopter A-Star equipped with GPS guidance and Accu-flo boom nozzles). In September 2017, herbicide was applied with the same helicopter deployment to another 267 ha in the Long Point Bay region. A further 329 ha were treated at a rate of 1200–3600 g glyphosate a.e./ha with 0.5–1.5% solution of Aquasurf® via ground application (Marsh Master™) (Robichaud and Rooney 2021c). In the winter, treated areas were rolled with a drum pulled by an Argo™ track vehicle or mowed using the Marsh Master™ to reduce the number of standing dead stems and enhance light penetration.
Our study focused on the portion of the Long Point peninsula comprising Crown Marsh and Long Point Provincial Park. Our experimental treatment sites were all situated in areas of Long Point marsh that had been treated with glyphosate-based herbicide and subsequently rolled or mowed the following winter. Portions of the invaded marsh were reserved as experimental controls (Fig. 1) and did not receive any treatment.
Long Point Peninsula (42° 34′ N, 80° 24′ W) is located on the north side of Lake Erie in Ontario. Net primary production measurements were taken at all sites, red symbols represent a subset of sites where decomposition measurements were taken. Triangles are herbicide-treated sites, circles are reference sites, and diamonds are P. australis-invaded sites. Image credit Matthew Bolding
Site establishment
To assess primary production, decomposition rates, and environmental conditions, we established 10 sites in un-treated P. australis habitat, 10 in herbicide-treated habitat, and 10 in uninvaded, reference habitat. Reference vegetation differs along the naturally occurring water depth gradient in the wetland, resulting in distinct plant communities of meadow marsh (n = 5) and emergent cattail marsh (n = 5). Meadow marsh occurs in areas of shallow standing water (May water depth < 40 cm) and saturated soil, where Calamagrostis canadensis (Canada bluejoint grass) is dominant, while emergent cattail marsh is in areas with deeper standing water (May water depth > 30 cm) and is dominated by a cattail species complex (Typha spp.), with a limited ecotone between the two plant communities occurring at intermediate water depths (May water depth 30–40 cm). Sample sites were situated to ensure that a parallel range of standing water depths was represented in each the three vegetation types (un-treated P. australis, herbicide-treated, and reference habitat). Water depths measured at the sample sites in May 2017 ranged from 14 to 56 cm (Table 1).
Primary production
To measure aboveground biomass, we collected live ramets, standing dead stems, and leaf litter of all species within three randomly established 0.25 m2 quadrats at each of the 30 sites (Fig. 1). Standing dead stems were considered any material that was still rooted and standing, while litter was any biomass that was no longer rooted. We collected this material July 22–25th 2017, during peak aboveground biomass (Yuckin and Rooney 2019). To measure belowground biomass, we used a modified soil ingrowth method (Neill 1992) at each site to measure the amount of biomass produced over the growing season (60–65 days). From 20 to 25 May 2017, we removed seven soil cores (4.8 cm diameter × 11.3 cm depth) at each site, extracted belowground plant biomass and replaced the lost volume with vermiculite. Vermiculite was chosen after pilot tests with potting soil and horticultural peat as it does not contain nutrients that could influence root growth into the soil cores. Soil cores were taken around the circumference of a 1-m-diameter circle centred on the quadrat to account for the heterogeneity in each site. Each soil core was retrieved during peak biomass (22–25 July). All root and rhizome matter from each core was collected. All the biomass from the above- and below-ground collections, including litter and standing dead, were dried for 48 h at 80 °C and then weighed. Aboveground biomass, litter, and standing dead were weighed to the nearest 0.01 g (Advanced Balance PB602-S, Mettler Toledo, ON, Canada), and belowground biomass was weighed to the nearest 0.0001 g (MS Precision Balance, Mettler Toledo, ON, Canada).
Decomposition rates
We performed a litter transplant experiment to separate the direct and indirect effects of P. australis invasion and herbicide-based control on decomposition rates. We deployed litter bags to measure decomposition rates at shallow (16.5 cm ± 10.8 SD), intermediate (26.7 cm ± 10.8 SD), and deep (34.1 cm ± 11.1 SD) water-depth sites in each habitat type (3 habitat types × 3 water depths = 9). Litterbags were made from 2-mm fibreglass mesh, to which we added 5.00 g (± 0.01 g; Advanced Balance PB602-S, Mettler Toledo, ON, Canada) of plant litter, which we collected from Long Point in August 2016 and air dried prior to storage: either P. australis leaves, Typha spp. leaves or C. canadensis leaves and stems. Calamagrostis canadensis stems were included in the litterbags because the growth form of C. canadensis did not allow for effective separation of the leaves from stems. The C:N ratio of all plant tissue was determined before deployment (Yuckin and Rooney 2019), and extra litterbags of each type were brought to the field as ‘travel blanks’ to calculate travel loss (Robertson et al. 1999).
From 12 to 14 May 2017, we deployed litterbags containing each type of litter at the nine shallow, medium, and deep-water sites, anchoring them to the substrate in clusters of three (one of each litter type). As standing dead plant material does not have contact with water or wetland substrate, we experimentally mimicked this condition. To do so, we tied additional litter bag replicates to poles at the three sites of intermediate water depth. These litter bag replicates stayed approximately one meter above the water surface throughout the experiment and are hereafter referred to as the “dry” treatment. Before litter bags were deployed in the field, we recorded the initial weight of litter in each bag. We collected three replicates of each litterbag type from each site after 2 weeks and then monthly until 15 May 2018. Upon retrieval, we dried the litter at 100 °C for 48 h before weighing it to the nearest 0.01 g (Advanced Balance PB602-S, Mettler Toledo, ON, Canada) to determine the mass loss, which we corrected for loss during travel using the travel blanks.
Environmental variables
To relate primary productivity and decomposition to environmental conditions, we measured water depth, water temperature, and photosynthetically active radiation at each site. To measure daily fluctuations in water depth and temperature, we installed data loggers (HOBO U20-001-02-Ti data logger, Onset, MA, USA) at each litterbag site (n = 9). Data loggers recorded from 14 June to 16 October 2017. In July 2017, we measured incident photosynthetically active radiation (PAR) (400–700 nm) and PAR extinction profiles at each site. Extinction profiles captured the % of incident PAR in 50-cm increments from the top of the canopy to the water or sediment surface. All PAR measurements were taken between 9:00 and 14:45 h on cloudless days in June, using two LI-190R LI-COR Quantum Sensors to simultaneously capture PAR above the canopy and within the canopy. Due to the low emergent vegetation cover and lack of canopy interception 100% of incident light reached the water surface, consequently extinction profiles were not measured at herbicide-treated sites.
Primary production data analysis
We considered five primary production response variables: (1) aboveground biomass, (2) belowground biomass, (3) ratio of belowground to aboveground biomass (analogous to root:shoot ratio, but with rhizomes and roots weighed jointly), (4) standing dead biomass, and (5) litter biomass. To test for differences in these variables among the habitat types and the water-depth gradient, we ran general linear models (ANCOVA) with habitat type (reference, un-treated P. australis, herbicide-treated) and water depth as predictor variables and included an interaction term. All response variables were square-root transformed to meet the assumption of normally distributed residuals. When an interaction was not significant, we interpreted the fixed factors independently and conducted Tukey’s post hoc test among the habitat types. Analyses were conducted using the MuMIn (Barton 2018) package in R (R Core Team 2016).
Decomposition rate data analysis
To calculate the decay rate of litter types in different water depths, we calculated the mass loss as: [(original mass loss − average travel loss for that plant species) − remaining mass]. We fit the percent mass lost over time using two models: the single exponential model (k) (Olson 1963) and the decaying coefficient model (k1) (Godshalk et al. 1978), as described by Ágoston-Szabó and Dinka (2008). Decay rates were determined using a nonlinear least-squares algorithm to fit the percent of dry-weight litter mass remaining over time using the Levenberg–Marquardt method in R. The decaying coefficient model (k1) is the most appropriate decay model for comparisons among different litter types and vegetation communities when the decay rate is expected to fluctuate. However, the single exponential model (k) is more common in the literature and allows for easier comparison to published studies (Dinka et al. 2004). As the decaying coefficient (k1) model is most appropriate for our purposes, we report it in the manuscript but report results from the single exponential model (k) in the appendices.
After calculating k and k1 over the deployment period, we averaged the values over time to generate one value per site. This value was our response variable and was used in a GLM to test if the single exponential decay rate (k) or the initial (k1) decay coefficient was predicted by species of litter (P. australis, Typha spp., and C. canadensis), site type (reference, P. australis invaded, and herbicide-treated), water depth (dry, shallow, intermediate, deep) and their interactions. We selected the general linear model with the lowest AICc value as the optimal model for each decay rate. The single exponential decay coefficient (k) was log-transformed, and the initial decay coefficient (k1) was square-root transformed to meet the assumption of normally distributed residuals. Decay rate function fitting was done using the minpack.lm (Elzhov et al. 2016) package in R (R Core Team 2016).
Environmental conditions data analysis
To characterize hydrologic conditions, we calculated the daily water-depth range, water-depth coefficient of variance, frequency intensity, and fluctuation intensity (sensu Trebitz 2006) for each site. We converted PAR to percent insolation passing through the canopy for analysis of light interception, with 100% indicating that all the incident PAR radiation penetrated the canopy to reach the water surface or sediment and 0% indicating that the canopy fully intercepted it. To determine if water-depth range, water temperature, and PAR interception differed among the plant communities, we ran three general linear models with water depth, water temperature, and percent PAR penetration as response variables and site type as the predictor variable. Daily water-depth fluctuations and daily water-temperature fluctuations were square-root transformed. Analyses were conducted using the MuMIn (Barton 2018) package in R (R Core Team 2016).
Results
Primary production
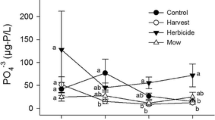
Primary production differed among the habitat types and along the water depth gradients in our study but was not predicted by an interaction between habitat type and water depth. Aboveground biomass was significantly lower in herbicide-treated sites (122 g/m2 ± 133 SD) compared to un-treated P. australis (1254 g/m2 ± 449 SD) and reference habitat (821 g/m2 ± 335 SD) and did not vary with water-levels (Fig. 2A, Table 2). Belowground biomass had a significant positive relationship with water depth, with the amount of belowground biomass increasing as water depths increased (slope estimate 0.261 ± 0.106 SE) (Fig. 2B; Table 2). Like aboveground biomass, aboveground:belowground biomass ratios were significantly lower in herbicide-treated sites (2.59 ± 5.30 SD) compared to un-treated P. australis (11.0 ± 4.39 SD) and reference habitat (14.7 ± 12.9). Aboveground:belowground biomass ratios also had a significant negative relationship with water depth (slope estimate − 0.052 ± 0.04 SE) (Fig. 2C, Table 2).
The amount of standing dead biomass differed significantly among all three habitat types. Herbicide-treated sites had the least standing dead biomass (18.7 g/m2 ± 22.4), as most had been removed via mechanical methods such as rolling or cutting. Reference marsh vegetation had an intermediate amount of standing dead biomass (808 g/m2 ± 839). In comparison, un-treated P. australis had significantly greater amounts of standing dead (1615 g/m2 ± 518) (Fig. 2D). Coinciding with the mechanical rolling and cutting, herbicide-treated sites had significantly more litter (1085 g/m2 ± 606) than un-treated (512 g/m2 ± 216) or reference vegetation (397 g/m2 ± 308) sites. Litter also had a significant positive relationship with water depth (slope estimate 0.598 ± 0.317 SE) (Fig. 2E). There was more dead aboveground biomass than live biomass (dead: live biomass) per meter-squared of wetland in un-treated P. australis (2.00 ± 1.04 SD). In contrast, herbicide-treated sites had very little aboveground biomass and > 47 times more dead plant material (47.39 ± 89.12 SD) than the other sites.
Relationships between primary productivity variables among habitat types and water depth gradient. There were significant differences among the habitat types for all aboveground biomass (a), Aboveground:belowground biomass (c), standing dead litter biomass (d) and litter biomass (e), but not for belowground biomass (b). Water depth was also a significant predictor variable for belowground biomass (b), belowground: aboveground biomass ratios (c), and litter (e). Shaded areas around the line of best fit represent 95% confidence intervals
Decomposition rates
By the end of the experiment, litter loss ranged from 5.75 to 74.65%, depending on the litter type and water depth (Fig. 3; Online Resource 3, 4). In general, submerged P. australis leaf litter decomposed the fastest, litter that was fully submerged also decayed significantly faster than litter in the “dry” treatment. Depending on water depth, the decomposition rate differed among site types (Table 3). In reference sites and P. australis-invaded sites, submerged litter decomposed fastest in deep water; in herbicide-treated sites, submerged litter decomposed fastest at intermediate water depths. Fully submerged litter also decayed significantly faster than litter in the “dry” treatment (Fig. 3). Decomposition rates also depended on the litter type, with P. australis leaf litter decomposing significantly faster than C. canadensis and Typha spp. (Fig. 3C, D; Table 3). The decaying coefficient model (k1) had a consistently better fit (R2 = 0.970 ± 0.002 SE; Table 1) than the single exponential (k) model (R2 = 0.819 ± 0.009 SE; Online Resource 1). The model that best predicted litter decomposition included an interaction between site type and water depth, as well as litter type, site type, and water depth as independent variables (Online Resource 2).
Change in percent mass remaining of submerged litter (a), change in percent mass remaining of standing “dry” litter (b), change in % mass remaining of C. canadensis, Typha spp. and P. australis litter in submerged sites (c), change in % mass remaining of C. canadensis, Typha spp. and P. australis standing “dry” litter (d), and change in mass remaining of standing “dry” and submerged litter (e) over 367 days (day 0: May 12–14, 2017). Note that error bars in a, c and e reflect the averaging of the three depths at which each litter type was submersed in each plant community. The lack of error bars in b and d reflects that the “dry” litterbags were deployed only in one of the three water depth stations
Environmental covariates
The daily water-level measurements in un-treated P. australis, herbicide-treated sites, and reference vegetation confirmed that relative depths of the shallow, intermediate, and deep-water treatments remained fairly consistent throughout the field season, although there was a general drawdown from August to October (Fig. 4). When stations were established in May, the different vegetation types were situated in water of equivalent depths, but on average water depths were deepest in un-treated P. australis sites (GLM F2,1122 = 61.600, p-value ≤ 0.001, RSE = 0.132, R2 = 0.099; Online Resource 5). When we considered daily fluctuations in water depth (daily maximum–daily minimum), there was a significant interaction between site type (un-treated P. australis, herbicide-treated, reference vegetation) and depth treatment (shallow, intermediate, and deep) (F8,1116 = 62.760, p-value ≤ 0.001, R2 = 0.310; Online Resource 6). Un-treated P. australis and reference vegetation drove this interaction—shallow and intermediate P. australis sites had significantly greater daily fluctuations compared to other vegetation types at the same depths, while deep-water reference vegetation sites had the greatest fluctuations compared to different vegetation types at the same depth (Online Resource 7, 8). In addition to having deeper water depths and greater daily fluctuations, un-treated P. australis sites also had the largest frequency intensity and coefficient of variation in water depths compared to herbicide-treated and reference vegetation (Online Resource 7).
A significant interaction between water-depth category and site type predicted daily water temperatures (F8,1116 = 61.710, p-value ≤ 0.001, R2 = 0.307; Online Resource 8) and daily water temperature fluctuations (F8,1116 = 369.000, p-value ≤ 0.001, R2 = 0.659; Online Resource 9). Average daily water temperature was positively correlated with water depth for the P. australis-invaded sites and reference vegetation communities, while herbicide-treated sites were warmer in shallower water. Herbicide-treated sites also had the greatest variation in daily water temperature fluctuations, likely because they have more open water, while P. australis-invaded sites had the smallest daily water temperature fluctuations (Online Resource 10). Relatively less PAR reached the bottom of the canopy in P. australis-invaded sites compared to reference plant communities, but this difference was not significant (F2,16 = 1.195, p-value = 0.328, R2 = 0.130; Online Resource 11).
Hourly water depths (m) averaged to daily values from June 14 to October 16, 2017, in nine sites in Long Point Provincial Park and Crown Marsh. Dashed lines are the deep-water sites, dotted lines are the intermediate water depth sites and solid lines represent the shallow water sites. Seiche events may account for the variation in daily water depths
Discussion
Invasive P. australis alters North American wetlands by displacing native plant communities with dense, tall monocultures. The establishment of P. australis has the potential to change carbon budgets directly by increasing the production of above- or below-ground biomass and indirectly by altering environmental conditions that control rates of decomposition. Best management practices to suppress P. australis include applying herbicide to kill the perennial plants and conducting a secondary treatment, such as rolling or mowing, to knock over standing dead stems. Despite the negative impacts of invasion on biodiversity, it is possible that P. australis effectively stores more carbon than the plant species it outcompetes. Further, those managing invasive P. australis may be interested in approaches that encourage decomposition of the extensive biomass produced by P. australis invasion to improve habitat quality for wildlife and speed the recovery of native plants. This topic constitutes a critical knowledge gap on the immediate effects of herbicide-based treatment of P. australis on carbon dynamics, as thousands of hectares of P. australis invaded marsh are treated with herbicide annually in North America (Hazelton et al. 2014). We are only beginning to consider this knowledge gap (e.g., Judd and Francoeur 2019).
We quantified the effect of P. australis invasion and herbicide-based P. australis suppression efforts on primary production and decomposition rates in a coastal marsh. Our results illustrate the large amount of aboveground biomass, including standing dead stems and litter, that results from P. australis invasion. This aboveground biomass turns into extensive amounts of litter following treatment. Managers seeking to remove this litter quickly may take advantage of increased decomposition rates by knocking down the stems to ensure they are submerged.
Invasion changes standing crop biomass
In reference plant communities, the cattail and meadow marsh vegetation that forms distinct zones along the water-depth gradient differ in their aboveground and total plant biomass. Our cattail marsh averaged over 1000 g/m2 in aboveground biomass, close to the 1200 g/m2 typical of P. australis. In contrast, on average, meadow marsh was less than 500 g/m2. Consequently, the effect of P. australis invasion on primary production depends on which reference community is being invaded (Yuckin and Rooney 2019). This limits generalizations regarding the effect of P. australis invasion on production in coastal marshes. Cattail marsh and P. australis-invaded marsh in the Great Lakes are much more productive than meadow marsh and are usually reported to have similar total and aboveground biomass (Rothman and Bouchard 2007; Duke et al. 2015; Yuckin and Rooney 2019). However, when P. australis replaces meadow marsh, there is a dramatic increase (~ 300%) in total biomass and a net increase in carbon sequestration.
Invasion causes changes to decomposition
The net effects of P. australis invasion on wetland decomposition rates are challenging to quantify because of its conflicting direct effects on litter chemical composition (e.g., reduced C:N ratio) and indirect effects on environmental conditions that influence decomposition (e.g., shading, cooling). Prior research with P. australis has not determined whether direct or indirect effects are more important: in some studies, environmental conditions were most important for decomposition (e.g., Duke et al. 2015), while in others, the litter composition was important (e.g., Windham 2001). In our study, environmental conditions exerted the largest influence on decomposition rates—specifically, whether litter was submerged or dry. Suspended litter (“dry” treatment), regardless of litter species or vegetation community in which it was deployed, decomposed considerably more slowly than submersed litter. The positive influence of submergence on decomposition has been documented in numerous studies: litter that is continually submerged decomposes faster than litter that is occasionally inundated (van der Valk et al. 1991; Dolinar et al. 2015; Vymazal and Březinová 2016), and occasionally, inundated litter decomposes faster than litter in consistently dry sites (Bedford 2005; Dolinar et al. 2015; Vymazal and Březinová 2016). These results highlight the importance of secondary treatment that will break P. australis stems and submerge them, increasing the pace of decomposition after herbicide treatment. This is likely desirable as removing the thatch of standing dead litter may help native seedlings establish (e.g., Bonello and Judd 2020) and speed recovery.
When considering only the submerged litter, differences between species were more apparent. As nutrient quality has a strong relationship with decomposition rate when litter is submerged (Enriquez et al. 1993), the high foliar nitrogen concentration in P. australis leaf litter (Farnsworth and Meyerson 2003; Hirtreiter and Potts 2012; Yuckin and Rooney 2019) suggests that it would decompose significantly faster than C. canadensis or Typha litter. Phragmites australis leaf litter did decompose fastest. Indeed, its initial decaying coefficient was about twice that of Typha spp. and C. canadensis tissues, which had similar nitrogen concentrations (Yuckin and Rooney 2019) and decomposed at equivalent rates. When we consider both primary production and decomposition, there is no significant change in total biomass when P. australis replaces cattail marsh. Still, the decomposition rate of submersed P. australis litter is faster than Typha spp. litter. It is possible then that P. australis invasion leads to a reduction in overall carbon storage when it displaces Typha. Despite no observed difference in aboveground or total standing crop biomass, the litter produced by P. australis decomposes at an initial decay rate twice as fast as that produced by Typha spp.
Unique environmental conditions in P. australis-invaded sites but no net effect on decomposition
Despite sites being selected based on common water levels in May, Phragmites australis-invaded sites retained deeper standing water than reference communities (particularly meadow marsh) later in the growing season. This contradicts studies that noted P. australis invasion reduces standing water depth due to increased losses from evapotranspiration or to infilling from increased belowground biomass or litter accumulation (Windham and Lathrop 1999; Rooth et al. 2003; Duke et al. 2015). In our study, we suspect water depths were deeper because P. australis sites have lower light penetration and water temperatures, thus reducing evaporation. However, these environmental conditions had a negligible effect on the decomposition of submersed litter. Changes that should increase decomposition rates (i.e., prolonged immersion in invaded sites) were offset by simultaneous changes that reduced decomposition rates (i.e., cooler, more stable water temperature and reduced light exposure in invaded sites). This combination of environmental changes caused by P. australis invasion offset one another, such that the consequent changes in litter quality dominated the net effect of invasion on decomposition rates in our study.
Response to herbicide treatment
The year after treatment, herbicide-treated sites had lower primary production than uninvaded reference vegetation. However, this difference may be short-lived. By July, approximately 10 months after herbicide treatment, live plant cover within the treated marsh averaged 41% (± 8.0 SD). Most of the plant community was composed of floating or submersed aquatic vegetation, with few emergent plant species. Submerged and floating plant tissues may decompose faster than emergent macrophytes (Chimney and Pietro 2006; Ping et al. 2017). This community change will likely alter decomposition and carbon sequestration rates in herbicide-treated marsh. Yet, it is difficult to assess the future trajectory of the plant community after only a single year. Passive recovery after P. australis suppression (i.e., allowing the native seed bank to re-establish unassisted) has been successful in some areas (Ailstock et al. 2001; Carlson et al. 2009; Baldwin et al. 2010), but there remains the possibility that P. australis or other undesirable species will establish (Hazelton et al. 2018; Robichaud and Rooney 2021a).
Environmental conditions play an essential role in plant community establishment. Secondary treatment increased light penetration to nearly 100% of incident PAR in some sites and increased the water temperature, which can benefit the general recovery of plant communities (Bonnewell et al. 1983; Leck 1996; Kettenring et al. 2006). Despite these advantages, the above-average Lake Erie water levels in 2017 and 2018 (e.g., Fisheries and Oceans Canada 2017, 2018) promoted the establishment of floating or submerged aquatic plant species rather than native species with emergent growth forms (Euliss et al. 2004). In addition to high water levels, the litter layer in herbicide-treated sites was sufficient to affect vegetation recovery. Rolling led to a thick mat or thatch of dead P. australis stems that will decompose faster than if the dead stems had been left standing but might impede seedling germination by changing environmental conditions, such as soil temperature or light availability, or by physically preventing new shoots from being able to push through the litter layer (Holdredge and Bertness 2011). Studies have demonstrated that removing P. australis litter results in increased species richness and native plant biomass recovery in the 1st year post-treatment (Ailstock et al. 2001; Carlson et al. 2009; Holdredge and Bertness 2011, Bonello and Judd 2020). Rolling or mowing standing dead litter to submerse it post-treatment will increase the pace of its decomposition. Still, burning or other practices that remove P. australis litter entirely may expedite recovery.
Conclusion
Land managers face trade-offs when considering whether to treat established P. australis populations. While biodiversity may increase (e.g., Markle and Chow-Fraser 2018; Polowyk 2020) following treatment, it is likely that carbon sequestration will be reduced in the short term. We found that suppression efforts dramatically reduced total biomass and carbon assimilation, at least in the 1st year post-treatment.
Despite increased water depth, water-level stability, and temperature in herbicide-treated sites, no increases in decomposition rates were observed relative to reference sites or P. australis-invaded sites. The similarities in decomposition rates across plant communities suggest that carbon stores might be unaffected despite the reduction in primary production. Importantly, our results indicate that litter decomposes much faster when submerged. These values provide evidence that land managers may need when deciding whether to perform secondary treatment when managing an invasive plant. Increasing the decomposition of P. australis litter by submerging or removing stems can aid the recovery of native plant species and reduce the competitive advantage of P. australis (Holdredge and Bertness 2011).
Plant community recovery is crucial for restoring carbon sequestration in herbicide-treated marsh. The rapid recovery of floating and submerged aquatic vegetation provides evidence that this gap in carbon sequestration services may be temporary. Provided that the recovering plant community shifts to an emergent marsh over time, carbon sequestration function should be recovered within the herbicide-treated marsh. However, this will depend on lake levels (van der Valk et al. 1994; Euliss et al. 2004) and the status of the seed bank (Ailstock et al. 2001; Carlson et al. 2009; Baldwin et al. 2010). Understanding these net effects on ecosystem functions provides necessary context for decision-makers who must consider societal values, economic feasibility, and other uncertainties (Liu et al. 2011; Martin and Blossey 2013), when deciding whether to treat established P. australis.
Data availability
The datasets used for analyses during the current study are available in a Figshare repository: primary productivity (https://doi.org/10.6084/m9.figshare.15067341.v1), decomposition data (https://doi.org/10.6084/m9.figshare.15067374.v1), photosynthetically active radiation (https://doi.org/10.6084/m9.figshare.15067686.v1) and site characteristics (https://doi.org/10.6084/m9.figshare.15067383.v1).
Code availability
Available upon request.
References
Ágoston-Szabó E, Dinka M (2008) Decomposition of Typha angustifolia and Phragmites australis in the littoral zone of a shallow lake. Biologia 63:1104–1110. https://doi.org/10.2478/s11756-008-0154-4
Ailstock MS, Norman CM, Bushmann PJ (2001) Common reed Phragmites australis: control and effects upon biodiversity in freshwater nontidal wetlands. Restor Ecol 9:49–59. https://doi.org/10.1046/j.1526-100x.2001.009001049.x
Alldred M, Baines SB, Findlay S (2016) Effects of invasive-plant management on nitrogen-removal services in freshwater tidal marshes. PLoS ONE 11:e0149813. https://doi.org/10.1371/journal.pone.0149813
Baldwin AH, Kettenring KM, Whigham DF (2010) Seed banks of Phragmites australis-dominated brackish wetlands: relationships to seed viability, inundation, and land cover. Aquat Bot 93:163–169. https://doi.org/10.1016/j.aquabot.2010.06.001
Barton K (2018) MuMIn: multi-model inference. R package version 1.40.4. https://CRAN.R-project.org/package=MuMIn
Bedford AP (2005) Decomposition of Phragmites australis litter in seasonally flooded and exposed areas of a managed reedbed. Wetlands 25:713–720. https://doi.org/10.1672/0277-521210.1672/0277-5212(2005)025[0713:DOPALI]2.0.CO;2
Bonello JE, Judd KE (2020) Plant community recovery after herbicide management to remove Phragmites australis in Great Lakes coastal wetlands. Restor Ecol 28(1):215–221. https://doi.org/10.1111/rec.13062
Bonnewell V, Koukkari WL, Pratt DC (1983) Light, oxygen, and temperature requirements for Typha latifolia seed germination. Can J Bot 61:1330–1336. https://doi.org/10.1139/b83-140
Carlson ML, Kowalski KP, Wilcox DA (2009) Promoting species establishment in a Phragmites-dominated great lakes coastal wetland. Nat Areas J 29:263–280. https://doi.org/10.3375/043.029.0306
Chambers RM, Meyerson LA, Saltonstall K (1999) Expansion of Phragmites australis into tidal wetlands of North America. Aquat Bot 64:261–273. https://doi.org/10.1016/S0304-3770(99)00055-8
Chimney MJ, Pietro KC (2006) Decomposition of macrophyte litter in a subtropical constructed wetland in south Florida (USA). Ecol Eng 27:301–321. https://doi.org/10.1016/j.ecoleng.2006.05.016
Christensen JR, Crumpton WG, van der Valk AG (2009) Estimating the breakdown and accumulation of emergent macrophyte litter: a mass-balance approach. Wetlands 29:204–214. https://doi.org/10.1672/08-27.1
Dinka M, Ágoston-Szabó E, Tóth. I (2004) Changes in nutrient and fibre content of decomposing Phragmites australis litter. Int Rev Hydrobiol 89:519–535. https://doi.org/10.1002/iroh.200410772
Dolinar N, Regvar M, Abram D, Gaberščik A (2015) Water-level fluctuations as a driver of Phragmites australis primary productivity, litter decomposition, and fungal root colonisation in an intermittent wetland. Hydrobiologia 774:69–80. https://doi.org/10.1007/s10750-015-2492-x
Duke ST, Francoeur SN, Judd KE (2015) Effects of Phragmites australis invasion on carbon dynamics in a freshwater marsh. Wetlands 35:311–321. https://doi.org/10.1007/s13157-014-0619-x
Ehrenfeld JG (2003) Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems 6:503–523. https://doi.org/10.1007/s10021-002-0151-3
Ehrenfeld JG (2010) Ecosystem consequences of biological invasions. Annu Rev Ecol Syst 41:59–80. https://doi.org/10.1146/annurev-ecolsys-102209-144650
Elzhov TV, Mullen KM, Spiess AN, Bolker B (2016) minpack.lm: R interface to the Levenberg–Marquardt nonlinear Least-Squares Algorithm found in MINPACK, plus support for Bounds. R package version 1.2-1. https://CRAN.R-project.org/package=minpack.lm
Enriquez S, Duarte C, Sand-Jensen K (1993) Patterns in decomposition rates among photosynthetic organisms: the importance of detritus C:N:P content. Oecologia 94:457–471. https://doi.org/10.1007/BF00566960
Euliss NH, Labaugh JW, Fredrickson LH et al (2004) The wetland continuum: a conceptual framework for interpreting biological studies. Wetlands 24:448–458. https://doi.org/10.1672/0277-5212(2004)024[0448:TWCACF]2.0.CO;2
Farnsworth EJ, Meyerson LA (2003) Comparative ecophysiology of four wetland plant species along a continuum of invasiveness. Wetlands 23:750–762. https://doi.org/10.1672/0277-5212(2003)023[0750:CEOFWP]2.0.CO;2
Findlay SEG, Dye S, Kuehn KA (2002) Microbial growth and nitrogen retention in litter of Phragmites australis compared to Typha angustifolia. Wetlands 22:616–625. https://doi.org/10.1672/0277-5212(2002)022[0616:MGANRI]2.0.CO;2
Fisheries and Oceans Canada (2017) Monthly water level bulletin: Great Lakes and Montreal Harbour. https://www.tides.gc.ca/C&A/bulletin-eng.html
Fisheries and Oceans Canada (2018) Monthly water level bulletin: Great Lakes and Montreal Harbour. https://www.tides.gc.ca/C&A/bulletin-eng.html
Godshalk GL, Wetzel RG, Kellogg WK (1978) Decomposition of aquatic angiosperms. II. Particulate components. Aquat Bot 5:301–327. https://doi.org/10.1016/0304-37707890074
Greenberg DA, Green DM (2013) Effects of an invasive plant on population dynamics in toads. Conserv Biol 27:1049–1057. https://doi.org/10.1111/cobi.12078
Hazelton ELG, Mozdzer TJ, Burdick DM, Kettenring KM, Whigham DF (2014) Phragmites australis management in the United States: 40 years of methods and outcomes. AoB Plants. https://doi.org/10.1093/aobpla/plu001
Hazelton ELG, Downard R, Kettenring KM, McCormick MK, Whigham DF (2018) Spatial and temporal variation in brackish wetland seedbanks: implications for wetland restoration following Phragmites control. Estuar Coasts 41:S68–S84. https://doi.org/10.1007/s12237-017-0289-z
Hirtreiter JN, Potts DL (2012) Canopy structure, photosynthetic capacity and nitrogen distribution in adjacent mixed and monospecific stands of Phragmites australis and Typha latifolia. Plant Ecol 213:821–829. https://doi.org/10.1007/s10530-010-9836-2
Holdredge C, Bertness MD (2011) Litter legacy increases the competitive advantage of invasive Phragmites australis in New England wetlands. Biol Invasions 13:423–433
IPCC (2021) Sixth Assessment Report. Working Group 1: the physical science basis. https://www.ipcc.ch/report/ar6/wg1/
Judd KE, Francoeur SN (2019) Short-term impacts of Phragmites management on nutrient budgets and plant communities in Great Lakes coastal freshwater marshes. Wetl Ecol Manag 27:55–74. https://doi.org/10.1007/s11273-018-9643-6
Kettenring KM, Gardner G, Galatowitsch SM (2006) Effect of light on seed germination of eight wetland Carex species. Ann Bot 98:869–874. https://doi.org/10.1093/aob/mcl170
Leck MA (1996) Germination of macrophytes from a Delaware River tidal freshwater wetland. Bull Torrey Bot Club 123:48. https://doi.org/10.2307/2996306
Lei C, Yuckin SJ, Rooney RC (2019) Rooting depth and below ground biomass in a freshwater coastal marsh invaded by European Reed (Phragmites australis) compared with remnant uninvaded sites at Long Point, Ontario. Can Field Nat 133:364–371. https://doi.org/10.22621/cfn.v133i4.2281
Liao CZ, Peng RH, Luo YQ et al (2008) Altered ecosystem carbon and nitrogen cycles by plant invasion: a meta-analysis. New Phytol 177:706–714. https://doi.org/10.1111/j.1469-8137.2007.02290.x
Liu S, Sheppard A, Kriticos D, Cook D (2011) Incorporating uncertainty and social values in managing invasive alien species: a deliberative multi-criteria evaluation approach. Biol Invasions 13:2323–2337. https://doi.org/10.1007/s10530-011-0045-4
Markle CE, Chow-Fraser P (2018) Effects of European common reed on Blanding’s turtle spatial ecology. J Wildl Manag 82:857–864. https://doi.org/10.1002/jwmg.21435
Martin LJ, Blossey B (2013) The runaway weed: costs and failures of Phragmites australis management in the USA. Estuar Coasts 36:626–632. https://doi.org/10.1007/s12237-013-9593-4
Mitsch WJ, Bernal B, Nahlik AM et al (2013) Wetlands, carbon, and climate change. Landsc Ecol 28:583–597. https://doi.org/10.1007/s10980-012-9758-8
Neill C (1992) Comparison of soil coring and ingrowth methods for measuring belowground production. Ecology 73:1918–1921. https://doi.org/10.2307/1940044
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–331. https://doi.org/10.2307/1932179
Ontario Ministry of Natural Resources and Forestry [OMNRF] (2016) Invasive Phragmites control at Long Point and Rondeau Provincial Park. Report, pp 1–18. http://rondeauprovincialpark.ca/wp-content/uploads/2019/09/MNRF-PhragER-Implementation-Plan-2019-v2.0-2019-08-14-FINAL.pdf
Ping Y, Pan X, Cui L et al (2017) Effects of plant growth form and water substrates on the decomposition of submerged litter: evidence of constructed wetland plants in a greenhouse experiment. Water 9:827. https://doi.org/10.3390/w9110827
Polowyk H (2020) Eleocharis geniculata Bent Spike-rush Great Lakes Plains population: conservation in the face of invasion. University of Waterloo, MSc Thesis. http://hdl.handle.net/10012/15948
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Robertson GP, Coleman DC, Bledsoe CS, Sollins P (1999) Standard soil methods for long-term ecological research. Oxford University Press, Oxford
Robichaud CD, Rooney RC (2017) Long-term effects of a Phragmites australis invasion on birds in a Lake Erie coastal marsh. J Great Lakes Res 43:141–149. https://doi.org/10.1016/j.jglr.2017.03.018
Robichaud CD, Rooney RC (2021a) Effective suppression of established invasive Phragmites australis leads to secondary invasion in a coastal marsh. Invasive Plant Sci Manag 14:9–19. https://doi.org/10.1017/inp.2021.2
Robichaud CD, Rooney RC (2021b) Differences in above-ground resource acquisition and niche overlap between a model invader (Phragmites australis) and resident plant species: measuring the role of fitness and niche differences in the field. Biol Invasions. https://doi.org/10.1007/s10530-021-02674-6
Robichaud CD, Rooney RC (2021c) Low concentrations of glyphosate in water and sediment after direct over-water application to control an invasive aquatic plant. Water Res 188:116573. https://doi.org/10.1016/j.watres.2020.116573
Rooth JE, Stevenson JC, Cornwell JC (2003) Increased sediment accretion rates following invasion by Phragmites australis: the role of litter. Estuaries 26:475–483. https://doi.org/10.1007/BF02823724
Rothman E, Bouchard V (2007) Regulation of carbon processes by macrophyte species in a great lakes coastal wetland. Wetlands 27:1134–1143. https://doi.org/10.1672/0277-5212(2007)27[1134:ROCPBM]2.0.CO;2
Schultz R, Andrews S, O’Reilly L, Bouchard V, Frey S (2011) Plant community composition more predictive than diversity of carbon cycling in freshwater wetlands. Wetlands 31:965–977. https://doi.org/10.1007/s13157-011-0211-6
Schummer ML, Palframan J, McNaughton E, Barney T, Petrie SA (2012) Comparisons of bird, aquatic macroinvertebrate, and plant communities among dredged ponds and natural wetland habitats at Long Point, Lake Erie, Ontario. Wetlands 32:945–953. https://doi.org/10.1007/s13157-012-0328-2
Tho BT, Sorrell BK, Lambertini C et al (2016) Phragmites australis: how do genotypes of different phylogeographic origins differ from their invasive genotypes in growth, nitrogen allocation and gas exchange? Biol Invasions 18:2563–2576. https://doi.org/10.1007/s10530-016-1158-6
Trebitz AS (2006) Characterizing seiche and tide-driven daily water level fluctuations affecting coastal ecosystems of the Great Lakes. J Great Lakes Res 32:102–116. https://doi.org/10.3394/0380-1330(2006)32[102:CSATDW]2.0.CO;2
van der Valk AG, Rhymer JM, Murkin HR (1991) Flooding and the decomposition of litter of four emergent plant species in a prairie wetland. Wetlands 11:1–16. https://doi.org/10.1007/BF03160837
van der Valk AG, Squires L, Welling CH (1994) Assessing the impacts of an increase in water level on wetland vegetation. Ecol Appl 4:525–534. https://doi.org/10.2307/1941954
Vilà M, Espinar JL, Hejda M et al (2011) Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol Lett 14:702–708. https://doi.org/10.1111/j.1461-0248.2011.01628.x
Vymazal J, Březinová TD (2016) Decomposition of Phragmites australis in relation to depth of flooding. In: Vymazal J (ed) Natural and constructed wetlands. Springer International Publishing, Cham, pp 57–68
Wilcox KL, Petrie SA, Maynard LA, Meyer SW, Erie Lake (2003) Historical distribution and abundance of Phragmites australis at Long Point, Lake Erie, Ontario. J Great Lakes Res 29:664–680. https://doi.org/10.1016/S0380-1330(03)70469-9
Windham L (2001) Comparison of biomass production and decomposition between Phragmites australis common reed and Spartina patens salt hay grass in brackish tidal marshes of New Jersey, USA. Wetlands 21:179–188. https://doi.org/10.1672/0277-5212(2001)021[0179:COBPAD]2.0.CO;2
Windham L, Lathrop R (1999) Effect of Phragmites australis common reed invasion on aboveground biomass and soil properties in brackish tidal marsh of Mullica River, New Jersey. Estuaries 22:927–935. https://doi.org/10.2307/1353072
Yuckin SJ, Rooney RC (2019) Significant increase in nutrient stocks following Phragmites australis invasion of freshwater meadow marsh but not of cattail marsh. Front Environ Sci 7:112. https://doi.org/10.3389/fenvs.2019.00112
Acknowledgements
This research was supported by Grants to R.C. Rooney from NSERC Discovery (RGPIN 03846) and Ontario Ministry of Natural Resources and Forestry non-consulting agreement MNRF-W-12-316 and to S. Yuckin from Mitacs Accelerate IT07466 73027 in partnership with the Nature Conservancy of Canada. We thank Heather Polowyk, Jessie Pearson, and Jacob Basso for support in the field and Dr. Janice Gilbert, Dr. Merrin Macrae, Dr. Roland Hall, and Dr. Marcel Pinheiro for valuable feedback on an early draft. We also thank Doug Wilcox for extremely helpful feedback on the submitted manuscript.
Funding
This work was supported by NSERC Discovery 03846 [Rooney], the Ontario Ministry of Natural Resources and Forestry MNRF-W-(12)6-17 [Rooney], and Mitacs Accelerate IT07466 73027 in partnership with the Nature Conservancy of Canada [Yuckin] and NSERC CGS-M [Yuckin].
Author information
Authors and Affiliations
Contributions
SJY and RCR conceptualized the study, RCR acquired funding, supplied resources, supervised and administered the project, provided validation and curated the data, SJY, CDR and GH conducted fieldwork, SJY and CDR conducted formal analysis and visualized the data, SJY wrote the original draft, and CDR, GH and RCR revised and edited the text.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
All authors consent to the publication of the manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yuckin, S.J., Howell, G., Robichaud, C.D. et al. Phragmites australis invasion and herbicide-based control changes primary production and decomposition in a freshwater wetland. Wetlands Ecol Manage 31, 73–88 (2023). https://doi.org/10.1007/s11273-022-09902-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-022-09902-3