Abstract
This paper presents the results of a project in which the seasonal changes in the chemistry of groundwater in dipwells at Wicken Fen National Nature Reserve were investigated for the first time. Thirteen dipwells arranged in two roughly perpendicular transects across Wicken Fen were sampled for groundwater on a monthly basis between April 2010 and April 2011. Surface water from a drainage ditch (Gardiner’s Drain) and groundwater from a further location (dipwell 17) were also sampled at the same interval. The water samples were analysed for pH, electrical conductivity (EC), major solutes (Ca, Na, Mg, S), nutrients (N, P, K) and trace elements (Al, Fe, Mn) in the laboratory. Measurements of pH and electrical conductivity were also taken from the soil at the fourteen dipwell locations. The results of these analyses are compared to borehole data, groundwater levels and precipitation data to form a new conceptual model of the spatial and temporal variations in groundwater chemistry at Wicken Fen, which have important implications for future hydrological and vegetation management regimes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wicken Fen in Cambridgeshire, UK is a National Nature Reserve, a Site of Special Scientific Interest (SSSI) and a RAMSAR site designated for its unique wetland habitat of international importance. Plant communities of UK national importance at the site include Phragmites australis (common reed)-Peucedanum palustre (milk-parsley) tall-herb fen (NVC S24) and Molinia caerulea (purple moor-grass)-Cirsium dissectum (meadow thistle) fen-meadow (NVC M24). These are accompanied at the site by several other fen meadow and tall-herb fen communities, and by carr (wet woodland), reedswamp and Cladium mariscus swamp (NVC S2) (Mountford et al. 2011).
The 323 ha reserve is run by the National Trust, a British conservation and heritage charity, and represents the last fragment of East Anglian Fenland habitat and has an especially rich flora and fauna, including a number of rare invertebrates. Since the millennium there have been concerns about falling groundwater levels and the adverse effect that this might have on wetland plant communities (McCartney and de la Hera 2004). However, a single dominant cause for this drying trend has not yet been identified.
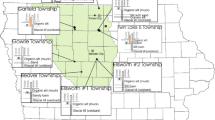
The fen is situated at c. 2 m Ordnance Datum and comprises up to 2 m of Holocene peat and lacustrine deposits underlain for the most part by bedrock comprising clays and mudstones of the Cretaceous Gault Formation (Boreham 2013). Wicken Fen is confined to the northeast by a low ridge (the Wicken ridge) capped by chalky glacial till, and to the northwest by a ridge of older bedrock strata (the Upware ridge) brought to the surface by an anticlinal structure. Run-off reaches Wicken Fen from the slopes of the Wicken ridge through several drains that flow intermittently. Wicken Fen comprises Sedge Fen to the east, underlain by impermeable mudstones of the Cretaceous Gault Formation, and Verrall’s Fen to the west, partly underlain by the Woburn Sands Formation (Boreham 2013). Although this sandstone is classified by the Environment Agency as a major aquifer with a potentially high yield, borehole investigations by the author suggest that its outcrop is hydrologically sealed by an overlying clay-rich periglacial deposit, so that it is unlikely to affect water tables in the fen. Wicken Fen is bounded to the south by Wicken Lode, a canalised water course leading to the River Cam at Upware. This paper presents the results of chemical analysis of groundwater from fourteen dipwells and from surface water in Gardiner’s Drain at Wicken Fen (see Fig. 1) for the period April 2010–April 2011 and provides an overview of hydrological processes at the site. It identifies for the first time a dense aquitard layer within the peat that supports surface water at several sites across the fen and disconnects it from the groundwater. This appears to permit water with very different chemistries to co-exist at different depths in many locations across the reserve. This has profound implications for the hydrological dynamics at the site and the management strategies for the fen itself.
Groundwater—dipwells
Thirteen dipwells arranged in two transects (one west-east and one south-north) across Wicken Fen, and one further eastern dipwell were sampled for groundwater at monthly intervals between April 2010 and April 2011 (13 occasions) (see Fig. 1). The water samples were analysed at the Geography Science Laboratories, University of Cambridge, for pH, electrical conductivity (EC) and major solutes (Ca, Na, Mg, S), nutrients (N, P, K) and trace elements (Al, Fe, Mn). The west-east transect comprised dipwells 1, 2, 4, 5, 6, and 14 extending across Verrall’s Fen and into the central part of Sedge Fen. The south-north transect across the central part of Sedge Fen comprised dipwells 8, 9, 10, 11, 12, 14, 15, and 16. Note that dipwells 3 and 7 were abandoned before this study commenced. Dipwell 17 was located in the eastern part of Sedge Fen near the Visitor Centre. The concentration of solutes recorded at this location far exceeded those from any other dipwells in this study, and it has been treated separately.
Surface water and soils
Samples from the water surface were taken with a bottle each month from a single site on Gardiner’s Drain (see Fig. 1) between April 2010 and April 2011. Soil samples were taken at the ground surface and at 15 and 20 cm depth at each dipwell site in April 2011. The soil samples were stored and analysed for pH and electrical conductivity in a similar way to the water samples.
Methods
Sampling and storage
Groundwater levels in dipwells were recorded on a monthly basis between April 2010 and April 2011. Water samples were extracted in the field from the top of the water column in each dipwell using a hand-pump and tube apparatus. Water samples were extracted into screw-top 250 cm3 plastic bottles. On return to the laboratory the water samples were stored in the dark at 4 °C prior to analysis, which was undertaken as soon as possible. In the laboratory 30 cm3 water samples were taken for pH and electrical conductivity (EC) analyses, 30 cm3 samples were taken for analysis of nitrate by ion sensitive electrode (ISE) and 15 cm3 samples were taken for analysis by Inductively Coupled Plasma-Atomic Emission Spectrometry (ICP-AES) (Perkin Elmer Optima 2100DV).
Soil samples were collected from the field using an Eijkelkamp ‘Dutch’ auger and trowel. The 100 cm3 soil samples were placed in sealed and labelled sample bags. On return to the laboratory the soil samples were stored in the dark at 4 °C prior to analysis. 10 cm3 of each homogenized soil sample was taken for pH and Electrical Conductivity (EC) analyses.
pH and electrical conductivity (EC) analyses
The pH and EC of the water samples was measured in the laboratory using calibrated probes using a paste of soil mixed with deionised water (1:2 ratio). The pH readings in this study were measured with a precision of 0.01 pH units. The EC readings were measured with a precision of 1µS/cm.
Inductively coupled plasma (ICP) analyses
Water samples were analysed by ICP-AES at 317.933 nm (calcium), 589.592 nm (sodium), (magnesium), 257.610, 181.975 nm (sulphur), 766.490 nm (potassium), 213.617 nm (phosphorus), 396.153 nm (aluminium), 238.204 nm (iron) and 285.213 nm (manganese). Emission spectra for three replicates from each sample were compared to those from a series of standards. In general the detection limit of the ICP-AES is at least 0.001 mg L−1 (ppm), although this varies with each element. Note that values for phosphorus have been converted to ortho-phosphate throughout this paper.
Nitrate analyses
Water samples were analysed for nitrate using an ion sensitive electrode (ISE). The mV values for each sample were compared to those from a series of nitrate standards. The precision of this technique is c. 0.1 mg L−1 (ppm).
Results
Figures 2, 3, 4, and 5 show the mean values (with one standard deviation) for the twelve groundwater chemistry determinands at dipwells along the two transects (W–E and S–N), together with data for the months with highest and lowest values for comparison. Table 1 shows the chemistry data (mean, maxima, minima) for both dipwell 17 groundwater and Gardiner’s Drain (GD) surface water throughout the study period. Where possible, values of these determinands are given in the text for local rainfall, although it should be noted that evapotranspiration is generally thought to increase ionic concentration of water from precipitation once in the ground by a factor of three.
Note that mudstones of the Gault Formation generally have elevated pore water concentrations of sodium, chloride and sulphate (Brightman et al. 1985). At shallower depths weathering of clay minerals also increases calcium and magnesium concentrations, and at the surface the chemical and microbial oxidation of iron sulphide often produces run-off with high electrical conductivity and raised acid sulphate loadings (Forster et al. 1995). In addition, analyses by Petch (1977) suggest that arable land on Gault Formation mudstones produces run-off with high nitrate values in the early winter months and following sustained precipitation. The main ionic solutes of the Woburn Sands Formation (Lower Greensand) groundwater include calcium, sodium and sulphate, which can be present in concentrations in excess of 100 mg L−1, with elevated nitrate and phosphate in some situations (Shand et al. 2003; Ander et al. 2004).
Overview of groundwater chemistry
pH values
In the W–E transect, there was a pattern of increasing pH towards the east. Dipwells 1, 2, and 4 in Verrall’s Fen all yielded minimum values below pH 6.3 in contrast to dipwells 5, 6, and 14 where maximum pH values exceeded pH 7.8 (see Fig. 2a). In the S–N transect, pH values were rather similar, with minimum values along the transect above pH 6.6 and maxima above pH 7.7 (see Fig. 3a). The most variable pH was at dipwell 4, which produced a range of 5.94–7.76. In contrast, the most stable pH was from Gardiner’s Drain with a range of 7.35–8.26 (see Table 1). For comparison, typical pH values for local rainwater are c. pH 5.1.
Electrical conductivity (EC)
In the W–E transect, dipwell 1 gave consistently low EC values (<1500 µS cm−1) compared to the extremely variable results from dipwells 2 and 4. The lowest EC recorded during for this transect was 93 µS cm−1 at dipwell 4, but the maximum was >2000 µS cm−1 at dipwell 2 (see Fig. 2b). Dipwells 5, 6, and 14 had less variation, but showed a pattern of increasing EC towards the east. In the S–N transect, EC values generally increased from south to north. Some of the highest EC values (>2500 µS cm−1) were observed at dipwell 15, yet low stable values (<1000 µS cm−1) persisted to the north at dipwell 16 (see Fig. 3b). Dipwell 17 consistently produced EC values above 2500 µS/cm throughout during the study and gave the highest recorded value (5352 µS/cm) (see Table 1). For comparison, typical EC values for local rainwater are c. 30 µS cm−1 (c. 90 µS cm−1 once affected by evapotranspiration in the ground).
Calcium (Ca)
In the W–E transect, dipwells 1, 2, and 4 in Verrall’s Fen all gave calcium values <40 mg L−1 at some point during the study, but produced quite variable results. In contrast, dipwells 5, 6, and 14 were more stable, but showed generally high calcium values (>150 mg L−1) towards the east (see Fig. 2c). In the S–N transect, calcium values generally increased from south to north. Calcium values were highest at dipwell 15 (>500 mg L−1), but lowest and most stable at dipwell 16 (<250 mg L−1) (see Fig. 3c). The most variable calcium values were observed at dipwell 17, which produced a range of 495–960 mg L−1 (see Table 1). For comparison, typical calcium values for local rainwater are <1 mg L−1.
Sodium (Na)
In the W–E transect, dipwells 1, 2, 4, and 5 in Verrall’s Fen all produced sodium values <10 mg L−1 at some point during the study. Dipwells 1 and 2 showed a large degree of variability with maxima up to 100 mg L−1, whilst dipwell 5 was more stable. Dipwells 6 and 14 also produced higher and more variable sodium values (see Fig. 2d). In the S–N transect, sodium values generally increased from south to north. Sodium values were highest at dipwell 15 (>120 mg L−1), but lowest and most stable at dipwell 16 (<50 mg L−1) (see Fig. 3d). The highest sodium value in the study was 227 mg L−1 from dipwell 17. In contrast, the lowest and most stable sodium values were from Gardiner’s Drain with a range of 12–44 mg L−1 (see Table 1). For comparison, typical sodium values for local rainwater are <2 mg L−1.
Magnesium (Mg)
In the W-E transect, dipwells 1, 2, 4, and 5 in Verrall’s Fen all gave magnesium values <4 mg L−1 at some point during the study, although dipwells 1, 2, and 4 were all very variable with maxima up to 22 mg L−1. In contrast, dipwells 5, 6, and 14 produced more stable results that generally increased from east to west (see Fig. 2e). In the S–N transect, magnesium values were quite stable and were highest (>25 mg L−1) at dipwells 12 and 15. Dipwell 16 produced the lowest and most stable magnesium values (<10 mg L−1) (see Fig. 3e). The highest magnesium value in the study was 146 mg L−1 from dipwell 17 (see Table 1). For comparison, typical magnesium values for local rainwater are <1 mg L−1.
Sulphur (S)
In the W–E transect, dipwells 1, 2, 4, 5, and 6 all gave sulphur values <1 mg L−1 at some point during the study. Dipwell 2 was certainly the most variable with sulphur values reaching a maximum in excess of 160 mg L−1. Dipwells 5, 6, and 14 produced more stable results that generally increased from west to east (see Fig. 2f). In the S–N transect, sulphur values were lowest and most stable at dipwells 8 and 16, and highest (>100 mg L−1) in the centre of the transect. Dipwell 16 produced the lowest and most stable magnesium values (<20 mg L−1) (see Fig. 3f). Dipwell 17 routinely produced sulphur values >500 mg L−1 during the study period and gave the highest sulphur value in the study (1159 mg L−1) (see Table 1). For comparison, typical sulphate values for local rainwater are <3 mg L−1, which is equivalent to < 1 mg L−1 sulphur.
Nitrate (NO3)
In the W-E transect, dipwells 1 and 5 gave the highest (>15 mg L−1) and most variable nitrate values. Dipwells 1, 2, 4 and 5 in Verrall’s Fen all gave nitrate values <2 mg L−1 at some point during the study (see Fig. 4a). In the S–N transect, maximum nitrate values and variability generally decreased from south to north (see Fig. 5a). The most variable nitrate values were at dipwell 8, which produced a range of 2–29 mg L−1. Gardiner’s Drain also produced nitrate values <1 mg L−1 during the study (see Table 1). Once released by microbial processes nitrate is not retained by fen soils and is easily released into groundwater. For comparison, typical nitrate values for local rainwater are <3 mg L−1. Wicken Fen is surrounded by extensive tracts of both arable and pastoral agriculture, which contribute greatly to the wet deposition of nitrogen at the site.
Phosphate (PO4)
In the W-E transect, phosphate was generally low (<4 mg L−1) and stable, except at dipwell 5 where values in excess of 20 mg L−1 were recorded (see Fig. 4b). In the S–N transect, dipwell 8 produced a large range of variation (2–29 mg L−1) compared to the low (<4 mg L−1) and stable values from dipwells 9 and 10 (see Fig. 5b). Phosphate was present at very low concentrations (<0.01 mg L−1) in many dipwells and in Gardiner’s Drain during the study period. The highest phosphate value in the study was 28 mg L−1 from dipwell 17 (see Table 1). Phosphate can be easily leached from anoxic fen soils, but is fixed by oxidised iron when water tables are low. However, these large spikes in phosphate may originate from spot eutrophication events caused by local animal activity. For comparison, typical phosphate values local rainwater are generally below 0.1 mg L−1 (Anthony et al. 2008).
Potassium (K)
In the W–E transect, potassium was generally low (<15 mg L−1) and stable, except at dipwell 5 where values in excess of 20 mg L−1 were recorded (see Fig. 4c). In the S–N transect, dipwells 11, 12 and 14 produced large maximum values (>15 mg L−1) and large variation compared to dipwells 8, 9, and 10 to the south and dipwells 15 and 16 to the north (see Fig. 5c). The highest potassium value was 57 mg L−1 from dipwell 17 (see Table 1). Potassium is often retained and stored by fen soils, but it can be released with high throughputs of water following prolonged precipitation. For comparison, typical potassium values for rainwater are <1 mg L−1.
Aluminium (Al)
In the W–E transect, aluminium values and variability generally decreased from west to east (see Fig. 4d). Dipwells 1, 2 and 4 in Verrall’s Fen all produced aluminium values >0.2 mg L−1 during the study. In the S–N transect, aluminium values were generally low (<0.1 mg L−1) and stable, except at dipwell 8 (see Fig. 5d). Aluminium was present at very low concentrations (<0.01 mg L−1) in many dipwells and in Gardiner’s Drain at some point during the study. Dipwell 17 gave some of the lowest (<0.001 mg L−1) and most stable aluminium values recorded (see Table 1). Aluminium is released as soil pH falls and becomes toxic to plants below pH 5. For comparison, typical aluminium values for local rainwater are generally far below 0.3 mg L−1.
Iron (Fe)
In the W–E transect, the was a large amount of variability in iron values except at dipwell 5 where they were consistently high (>0.25 mg L−1) (see Fig. 4e). In the S–N transect, the variability of iron was also high, except at dipwells 12, 15, and 16 where low (<0.1 mg L−1) and stable values persisted (see Fig. 5e). The highest iron value recorded was >2 mg L−1 from dipwell 17 (see Table 1). For comparison, typical iron values for local rainwater are far below 0.1 mg L−1.
Manganese (Mn)
In the W-E transect, the was a large amount of variability in manganese values, especially at dipwells 2 and 6 where concentrations exceeded 0.5 mg L−1 (see Fig. 4f). In the S–N transect manganese values (>0.5 mg L−1) and variability were highest in the centre of the transect, and lowest (<0.2 mg L−1) at dipwells 8 and 16 (see Fig. 5f). Manganese was present at very low concentrations (< 0.01 mg L−1) in many dipwells and in Gardiner’s Drain at some point during the study. The highest manganese value was 1.91 mg L−1 from dipwell 17 (see Table 1). In general, manganese concentrations are thought to increase with low soil pH, and below pH 5.5 manganese becomes extremely soluble. Soil anoxia due to winter water-logging may also increase soluble manganese ions. Above pH 7.5 manganese availability may become a limiting factor for plant growth. For comparison, typical manganese values for local rainwater are far below 0.1 mg L−1.
Correlations within the data
Within the entire thirteen month data set there was a strong positive correlation (r2 > 0.6) between Electrical Conductivity (EC) and calcium values, and between EC and magnesium values. This is probably because calcium and magnesium ions make a large contribution to the total solute loading, which in turn strongly influences EC. There was also a strong positive correlation (r2 > 0.6) between calcium and magnesium values, and between calcium and sodium values. The relationship between magnesium and sodium was not as robust (r2 > 0.5), but these three alkali and alkaline earth metal ions tend to co-vary together in groundwater systems. It is notable that potassium does not show any significant relationship with calcium, magnesium or sodium. There is a positive correlation (r2 > 0.5) between potassium and phosphate. This is interesting, because both nutrients are part of generic ‘NPK’ agricultural fertilisers. However, it is notable that neither potassium nor phosphate shows any significant relationship to nitrate values.
Groundwater levels, rainfall and water chemistry
Groundwater levels
The mean values (with one standard deviation) for groundwater levels at dipwells along the two transects (W–E and S–N), together with values for the highest and lowest months for comparison are shown in Fig. 6a, b. These data clearly show high water levels, at or above the ground surface in March 2010, but low groundwater levels in July 2010. The typical annual excursion of groundwater levels is around 60 cm. In general, dipwells in the centre of the fen away from ditches and drains appear to have higher spring water tables, but those adjacent to ditches and drains seem to maintain higher levels of summer groundwater. This supports the conceptualisation of McCartney and de la Hera (2004).
Groundwater levels—mean values (with one SD) and data for the months with the highest and lowest values for a dipwells in the west–east transect and b dipwells in the south-north transect. Groundwater levels—values for c three selected dipwells (11, 12, and 16), d mean values (with one SD) calculated from all dipwells and e monthly change in groundwater levels. Rainfall—f monthly totals for precipitation at Wicken Fen throughout the study period
Figure 6c shows groundwater levels for three selected dipwells (11, 12, and 16) throughout the study period. Dipwell 11 close to Gardiner’s Drain does not have water at or close to the surface at any point during the year. In contrast, dipwell 12 in the centre of the fen does have groundwater at or above the surface during the winter and spring months. Dipwell 16 close to the northern fen margin shows one of the smallest excursions of groundwater (c. 40 cm) in the study. The mean monthly values (with one standard deviation) for groundwater levels at all dipwells throughout the study period are shown in Fig. 6d. It is remarkable how similar and synchronous the changes in dipwell water levels across the fen are. The annual cycle of groundwater excursion 2010–2011 can be clearly seen, with maxima in spring (February–April) and minima in summer (July–August). The water level change (rise and fall) between months (see Fig. 6e) indicates that the maximum drawdown was in July 2010, but that there was a strong increase in water levels by September 2010. For comparison, monthly rainfall for the study period is shown in Fig. 6f. The rainfall for August 2010 was a little higher than usual and so it is likely that in some years groundwater levels would remain somewhat lower throughout September and October than observed in the 2010–2011 1 cycle.
Correlations between groundwater levels and water chemistry
The level of water in Gardiner’s Drain was not specifically measured during the study, but dipwell 10 provides the nearest location as an approximation. It is known that water levels in Gardiner’s Drain and nearby dipwells co-vary to a large extent (McCartney and de la Hera 2004). There is a clear relationship between EC in Gardiner’s Drain and water level at dipwell 10 (r2 > 0.5). High water levels in spring seem to lower the concentration of ionic solutes, whilst low summer water levels appear to produce higher EC. In general, this could be explained by rainwater-dominated run-off in the spring, and groundwater dominated flow in the summer months. Indeed, EC at Gardiner’s Drain was also equally well correlated with water levels at many dipwells suggesting that this is a site-wide relationship. It is clear that the interaction between precipitation, evapotranspiration, soil and surface water is a strong factor in determining the chemistry of groundwater at Wicken Fen (Grieve et al. 1995).
In contrast, water levels at dipwell 1 in Verrall’s Fen correlated with very little in the chemistry. The most significant correlation was with potassium (r2 > 0.4), hinting that concentrations might increase with falling water level. The same was true for dipwell 2 where again the best correlation was with potassium (r2 > 0.5). Dipwell 4 showed a correlation (r2 > 0.5) between falling water levels and higher EC and magnesium (r2 > 0.5), and between falling water levels and higher calcium and potassium (r2 > 0.4). This suggests the influence of solute-rich groundwater in summer and low ionic strength precipitation in spring, in a similar fashion to the surface water in Gardiner’s Drain.
The water levels in dipwells 5 and 6 in the centre of the fen correlated with very little. However, in dipwell 5 there was a hint that manganese concentrations increased with rising water levels (r2 0.4), and in dipwell 6 a suggestion that calcium concentrations increased as water levels rose (r2 > 0.4). These observations imply that in some locations, certain ions are associated with surface run-off rather than deeper groundwater.
In the southern part of Sedge Fen adjacent to Wicken Lode, falling water levels in dipwell 8 showed a good correlation with increasing potassium (r2 > 0.7). This is a similar to the pattern observed at dipwells 1, 2 and 4 on Verrall’s Fen. In contrast, water levels at dipwell 9 correlated with very little apart from increasing manganese concentrations with rising water levels (r2 > 0.5), again hinting that manganese ions may be associated with surface run-off rather than deeper groundwater.
Rising water levels at dipwell 10 adjacent to Gardiner’s Drain correlated well with increased calcium and manganese values (r2 > 0.6), suggesting that these ions may be associated with surface run-off in the spring. However, water levels at dipwells 11, 12 and 14 apparently correlated with very little in the chemistry, perhaps because they receive little or no run-off. In contrast, dipwell 15 in the northern part of Sedge Fen showed a strong correlation between increased calcium and sodium concentrations and rising water levels (r2 > 0.6). The same pattern was observed at dipwell 16 near Spinney Bank, where higher calcium values exhibited a strong correlation with increasing water levels (r2 > 0.7). Sodium also showed similar behaviour, although the strength of the relationship was slightly weaker (r2 > 0.6). These patterns again appear to be associated with surface run-off in the spring.
Dipwell 17 in the eastern part of Sedge Fen has been treated separately because of its elevated ionic solute levels (Table 1). At this site there was a good correlation between rising water levels and increasing sulphur concentrations (r2 > 0.6) and a weaker relationship between low water levels and elevated calcium values (r2 > 0.4).
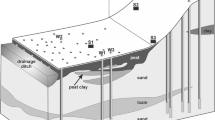
Figure 7 presents a conceptual model that summarises the differences in groundwater chemistry observed between high groundwater levels in spring and low groundwater levels in summer. Elevated calcium and manganese in high spring groundwater appears to reflect insularity, whilst high solute levels in low summer groundwater seem to be related to proximity and connection to Wicken Lode and the system of drains.
Conceptual groundwater chemistry model for Wicken Fen showing the main changes between spring high groundwater levels and summer low groundwater levels. Note that high solute values in spring suggest insularity, whilst high solute values in summer suggest connectivity to Wicken Lode and the system of drains
Relationship between soil and groundwater pH and electrical conductivity (EC)
Soil pH and EC have been measured at each dipwell site from samples taken at the ground surface, 15 and 25 cm depth in April 2011, and are compared to mean values of pH and EC from groundwater at the same sites (see Fig. 8a–d). In the W–E transect for several dipwells there is a marked increase in pH values at the soil surface compared to those from deeper in the soil and from the groundwater (see Fig. 8a). Soil surface EC exhibits an approximately inverse relationship with soil surface pH, so that the most alkali soils appear to be the lowest in ionic solutes (see Fig. 8b). This curious relationship may be due to evaporation of surface water forming a localized alkaline upper soil horizon. It is clear that to the east of the W–E transect, groundwater EC values increase relative to the soil values. In the S–N transect, soil surface pH is only slightly elevated compared to values for groundwater and those deeper in the soil (see Fig. 8c). However, for much of the S–N transect groundwater EC substantially exceeds values from the soil samples (see Fig. 8d).
Discussion
Surface water chemistry in Gardiner’s Drain
McCartney et al. (2001) have suggested that in winter and spring, water flow in drainage ditches (including Gardiner’s Drain) is largely derived from high water tables in the fen, but that in normal summers, the movement of water is largely from the ditches down into the groundwater. This presumes that in summer there is a flow from elsewhere feeding into the ditches, which keeps the groundwater in their vicinity elevated (zone 3 in the conceptualisation of McCartney et al. 2001). However, August 2010 appears to have been a little wetter than usual, and it is expected that there would be a net outflow of water from the fen into the drains and ditches in this situation (McCartney and de la Hera 2004).
High water levels in Gardiner’s Drain seem to lower the concentration of ionic solutes, whilst low summer water levels appear to produce higher EC. In general, this could be explained by rainwater-dominated run-off in the winter and spring, and groundwater-dominated flow in the summer months. However, rainwater in this area is expected to have a pH of c.5.6 and an electrical conductivity <50 µS cm−1, whereas the lowest EC in the Gardiner’s Drain water was >800 µS cm−1 and the lowest pH was 7.37. Even the episodes of lowest ionic solute concentration are clearly not simply rainfall, but are the result of modification by contact with fen soils or soils on the Wicken ridge.
The calcium-magnesium-sodium chemistry of the water in Gardiner’s Drain suggests that it has been in contact with at least the upper parts of the fen soil, probably including the dense marl layer identified by Rowell (1997) and verified by the author from boreholes at c.20–30 cm depth across much of the site.
Gardiner’s Drain had three main phases of calcium-rich high-pH water in summer 2010 (May–July), autumn 2010 (November) and spring 2011 (March–April) which do not correlate strongly with either the groundwater levels in nearby dipwells or indeed with rainfall records. The annual cycle of changing water chemistry in Gardiner’s Drain can also be visualised in the principal component analysis correlation plot of scores and vectors (Fig. 9). Months with similar groundwater chemistries plot together. The co-variance of vectors calcium and sodium, and the anti-phase relationship of vectors manganese and magnesium can also be seen. Thus it is clear that there are further cryptic factors such as run-off from the Wicken ridge or and unidentified source of groundwater at work influencing the water chemistry in Gardiner’s Drain.
There was a flush of phosphate in June 2010 and another of nitrate and potassium in September 2010. Nitrate was high in winter and spring, with a flush of potassium in March 2011. Nitrate is not held by soils and is readily leached into watercourses. Phosphate is also very soluble, but is adsorbed by iron III complexes in the soil. However, phosphate can be released when anaerobic conditions convert oxidised iron III into soluble reduced iron II. In contrast, potassium is readily adsorbed and held by soils, but it can increase with higher water flows as it dissolves from entrained suspended sediment. Low nitrate in Gardiner’s Drain appears to be associated with falling water tables and perhaps uptake by plants during the spring and summer, but higher nitrate seems to be linked to rising water levels in the autumn and winter as precipitation drains through decaying vegetation and fen soils. The small release of phosphate in June 2010 may also be associated with falling water levels. The flush of potassium into Gardiner’s Drain may follow months with high rainfall totals.
Groundwater chemistry in the west–east dipwell transect
Western Verrall’s Fen (dipwells 1, 2 and 4)
Dipwells 1, 2 and 4 were all more than 50 m from ditches and drains, and thus expected to be unaffected by them (zone 4 in the conceptualisation of McCartney et al. 2001). However, the conceptual model (Fig. 7) suggests behaviour more similar to sites connected to the system of drains rather than insular behaviour.
All three dipwells produced some of the most acid (<pH 6.2) groundwater recorded in the study period. They all also showed wide variations in ionic solutes, although these changes were often out of phase with each other, indicating some very different factors affecting each location. This is best illustrated by the calcium values which decreased throughout the study at dipwell 1, increased throughout the study at dipwell 2 and peaked in the middle of the study at dipwell 4, at least showing some correlation with fluctuating groundwater levels.
Similar erratic behaviour was observed in nitrate, phosphate and potassium levels. There were lower nitrate levels in spring and summer associated with falling water tables and higher nitrate in the autumn and winter related to rising groundwater levels. In general, potassium peaked in the summer and appeared to be linked to low groundwater levels. Phosphate levels peaked in the winter and nitrate increased throughout the study. These changes are difficult to understand, but are perhaps linked to decomposition of organic material during a mild autumn and winter, which subsequently liberated nitrate and phosphate into the high water table.
In addition, sulphur levels were much higher at dipwell 2 than the other two locations. The extremes of ionic solute concentrations recorded in these dipwells presumably equate to rainwater dominated (low pH and EC) and deeper groundwater dominated (high pH and EC) influences. The proximity of dipwells 1 and 2 to each other further compounds the conundrum of the anti-phase relationships observed. One explanation could be that pulses of water with different chemistries move laterally through the fen, sequentially affecting different dipwells. It is clear that the groundwater chemistry across this part of Verrall’s Fen is very dynamic.
Eastern Verrall’s Fen and Western Sedge Fen (dipwells 5, 6, and 14)
Dipwells 5, 6, and 14 were all more than 50 m from ditches and drains, and thus expected to be unaffected by them (zone 4 in the conceptualisation of McCartney et al. 2001). The conceptual model (Fig. 7) suggests insular behaviour for dipwells 5 and 6.
The dipwells all maintained groundwater pH >6.5, but in general showed a pattern of increasing ionic solutes from west to east. However, there were considerable variations in ionic solutes over time at each location, and although some ions loosely co-varied, others certainly did not. Dipwell 5 showed extremes of ionic solute concentrations that probably equate to rainwater dominated (low pH and EC) and deeper groundwater dominated (high pH and EC) influences. Dipwells 6 and 14 showed no such association, although at dipwell 6 there was some suggestion that elevated calcium concentrations were linked to higher groundwater levels.
Dipwell 5 exhibited a significant flush of nitrate, phosphate and potassium in autumn and winter, whereas dipwell 6 had flushes of potassium and phosphate in the summer and autumn, and nitrate peaked in the winter. In contrast, there was a strong spring flush of potassium at dipwell 14. At dipwell 5 there were lower nitrate levels in spring and summer associated with falling water tables and higher nitrate in the autumn and winter related to rising groundwater levels. Dipwells 6 and 14 did not show these patterns and appeared to behave quite differently to each other. The complexity of groundwater conditions is illustrated by the behaviour of sulphur at these dipwells, where anti-phase relationships were observed, particularly between dipwells 6 and 14. This again hints that pulses of water with different chemistries may move laterally through the fen, affecting different dipwells at different times. The spatially patchy and temporally changeable nature of groundwater chemistry is a clear characteristic of this part of Wicken Fen.
Groundwater chemistry in the south–north dipwell transect
Southern Sedge Fen (dipwells 8, 9, 10, 11, and 12)
Dipwell 8 was within 50 m of Wicken Lode and therefore expected to be affected by it (zone 2 in the conceptualisation of McCartney et al. 2001). This is behaviour is confirmed by the conceptual model (Fig. 7). Dipwells 10 and 11 were both within 50 m of Gardiner’s Drain and so expected to be affected by it (zone 3 in the conceptualisation of McCartney et al. 2001). Dipwells 9 and 12 were both more than 50 m from ditches and drains, and thus expected to be unaffected by them (zone 4 in the conceptualisation of McCartney et al. 2001). However, the situation shown by the conceptual model (Fig. 7) suggests dipwells 9 and 10 to be insular and dipwells 11 and 12 to have no overall pattern.
The dipwells all maintained a groundwater pH >6.5, often increasing to values close to pH 8 in November 2010. Dipwell 8 showed a pattern of broadly increasing ionic solutes over the study period, although the concentrations of the nutrients nitrate, phosphate and potassium all appeared to decrease between autumn 2010 and spring 2011, apparently linked to rising water levels. Dipwells 9 and 10 were very similar in that they maintained variable but modest ionic solute values over the study period. Rising water levels were linked to increasing calcium concentrations at dipwell 10. Phosphate was generally low at both locations, but lower nitrate in spring and summer was probably related to falling water tables and higher nitrate in the autumn and winter was associated with rising groundwater. The uptake of nitrate in the spring and summer by plant growth, and its release during decomposition in the autumn and winter could play an important role in understanding these patterns.
Dipwells 11 and 12 both exhibit a pattern of increasing ionic solutes over the study. A similar pattern was followed by nitrate concentrations. A flush of potassium appeared to be associated with falling summer water levels. Dipwell 11 showed a flush of phosphate in winter, although this was not detected in dipwells 10 and 12. The pattern of generally increasing solutes (particularly sodium, calcium and magnesium) from south to north along this transect is clear. Superimposed on this are variations in nutrients that sometimes, but not always related to changes in groundwater levels.
Northern Sedge Fen (dipwells 14, 15, and 16)
Dipwells 14 and 15 were both more than 50 m from ditches and drains, and thus expected to be unaffected by them (zone 4 in the conceptualisation of McCartney et al. 2001). Dipwell 16 was within 50 m of Spinney Bank drain and therefore expected to be affected by it (zone 1 in the conceptualisation of McCartney et al. 2001). However, the conceptual model (Fig. 7) suggests that dipwells 15 and 16 are insular, and that dipwell 14 has no overall pattern.
The dipwells all maintained a groundwater pH >6.5, but dipwell 16 produced a value in excess of pH 8 in November 2010. Dipwells 14 and 15 showed a pattern of broadly increasing ionic solutes over the study period, but dipwell 16 had much lower and more stable concentrations of magnesium, sodium and sulphur. However, higher concentrations of calcium at dipwells 15 and 16 were strongly linked to rising groundwater levels. There was spring flush of potassium at dipwell 14 in 2010 perhaps linked to falling groundwater, and a summer flush of nitrate at dipwell 15, presumably related to rising water tables. Curiously, nitrate appeared to slowly increase over the study period at dipwell 16, whilst potassium slowly decreased. All three dipwells showed a small flush of phosphate in June 2010. Again, the somewhat chaotic nature of NPK in the groundwater chemistry is clear.
Groundwater chemistry at dipwell 17
Dipwell 17 is more than 50 m from ditches and drains, and thus expected to be unaffected by them (zone 4 in the conceptualisation of McCartney et al. 2001). This is insularity is confirmed by the conceptual model (Fig. 7).
The groundwater at dipwell 17 had an unexceptional neutral to slightly alkaline pH in the range 6.9–7.8. It is the elevated ionic solute loading that separates this ‘hot spot’ location from all the others considered in this study (see Table 1). Calcium, magnesium, sodium and sulphur values were all often an order of magnitude greater than in other neighbouring dipwells. The same was true for both iron and manganese values. In contrast, nitrate, phosphate and potassium were often no higher than other dipwells in Sedge Fen. It is clear that there are some special circumstances surrounding the groundwater chemistry in this eastern part of the fen. There is some evidence from borehole data that a particular configuration of fen deposits and hydrology at the fen-edge might account for these unusual patterns (Boreham 2011). The pattern of variation of calcium ions over time, in particular, seems to be in anti-phase with those from other Sedge Fen dipwells, with high rather than low values in the summer, loosely linked to low water tables. Rising water levels appeared to be associated with increased sulphur concentrations at the site, perhaps indicating that oxidizing sulphides in the upper part of the soil contribute to this loading. Potassium and phosphate values, although elevated in spring 2010, rapidly decreased and remained low for much of the study. Nitrate values increased during the summer showing the opposite relationship to low water tables observed elsewhere in Sedge Fen.
Conclusions
It appears that the pattern of varying precipitation and water tables at Wicken Fen in the period April 2010–April 2011 were not necessarily typical of the recent past. In particular, the slightly higher rainfall in August 2010 led to higher than usual groundwater levels in the autumn, compared with data from previous years. It is perhaps for this reason that some ionic solutes showed increasing values over the study period, rather than the purely cyclical patterns that might have been expected. It is not obvious whether these patterns reflect part of a longer-term trend, a fluctuation over several years or a short-lived aberration. Some changes in groundwater chemistry over the study period can be linked to seasonally changing water levels, hinting at different chemistries for deep groundwater and surface-influenced water. Other changes seem to be so short-term and sporadic that it is difficult to make sense of the variations, although they might hint at influx from groundwater sources not directly derived from precipitation. Some dipwells appear to co-vary together in many respects, whilst other dipwells close together appear to differ wildly or even vary in anti-phase to each other.
Multivariate statistics (cluster analysis and principal component analysis (PCA) have also been used by the author to disentangle some of the less-obvious relationships within the data (see Fig. 9). As an example, Fig. 10 presents a dendrogram showing the results of agglomerative cluster analysis on the mean water chemistry data throughout the study period. Although this removes the temporal component from the data, it is clear that the dipwell sites can be divided into three groupings or clusters with least dissimilarity. Dipwell 17 stands out on its own due to its unusually elevated ionic solute concentrations. Dipwells 9, 10, 11, 12, 14, and 15 cluster together in terms of average groundwater chemistry and are adjacent to each other in the south-north transect across Sedge Fen. The remaining cluster groups together dipwells from Verrall’s Fen (1, 2, 4, 5), marginal sites (6, 8, 16) and Gardiner’s Drain. Tools such as this can be very useful in divining patterns within difficult and complex data sets such as this.
It is clear that the dipwells on Verrall’s Fen (1, 2, 4) are characterised by their lower pH values and relatively elevated aluminium levels, and by potassium-rich deep groundwater that apparently becomes diluted as surface precipitation raises water tables in the winter and spring. In contrast, dipwells in the northern part of Sedge Fen (12, 14, 15) are typified by elevated calcium, sodium, magnesium and sulphur levels, which increase with rising water levels. Between these two extremes were dipwells in the central and southern part of Sedge Fen (9, 10, 11), and the water in Gardiner’s Drain that often showed increased calcium and manganese levels associated with high water tables. It is perhaps no surprise that dipwell 8 adjacent to Wicken Lode shows more moderate variations in ionic solutes than some other dipwells on Sedge Fen. Similarly, dipwell 16 adjacent to Spinney Bank drain seems to be insular and almost cut off from the variations experienced in the centre of Sedge Fen.
Groundwater at dipwell 17 also had a calcium, sodium, magnesium and sulphur dominated chemistry, but with hugely elevated values compared to other dipwells in the study. An added complexity in understanding the dynamics of the groundwater chemistry at this location was the discovery of an impermeable layer of dense and relatively dry peat, at c.20 cm depth, which acts as an aquitard holding up surface water (Rowell 1997). It is not known precisely how common this ‘aquitard layer’ is in Wicken Fen, but many boreholes and analyses by the author in September 2011 showed that a dense dry layer in the fen soil was present at 20–30 cm depth near dipwell 5 on Verrall’s Fen and at a similar depth near dipwell 12 on Sedge Fen and in many other locations. The ‘aquitard layer’ at this depth generally has both an increased calcium carbonate and a decreased organic content, and is stratigraphically equivalent to an equally dense marl layer encountered across large areas of the fen.
The dense peat and marl has the effect of causing autumn and winter precipitation to pool above the aquitard, effectively slowing the percolation of surface water downwards towards the deeper groundwater (cf. Cook and Harding 2005), and allowing water with very different chemistries to co-exist at different depths in many locations across the site. It also has the important effect of effectively disconnecting the fen vegetation communities from the deeper groundwater for much of the year. Thus across Wicken Fen it seems that nationally important plant communities, and especially the dominant Phragmites-Peucedanum tall-herb fen (NVC S24) are largely reliant on the edaphic conditions and hydrology in the top c.30 cm of soil above the aquitard (see Fig. 8a–c).
This is rather different to the model proposed by McCartney et al. (2001), where it was presumed that standing surface water on the fen in winter was caused by saturated peat throughout the soil profile. The functional difference between the two models is that in the former, the lower (deep groundwater) water table and the upper surface water level may vary independently and might not be as closely linked as previously assumed.
It is clear that the separation of lower ‘deep groundwater’ and upper ‘precipitation derived’ surface water by an aquitard might explain the huge seasonal variations in ionic solutes observed in some dipwells (e.g. 1, 2, 4, 5, 8). Equally, the slow passage of surface water through a calcium carbonate-rich marl layer at 20–30 cm depth would explain the elevated calcium, magnesium and sodium observed at some dipwells (e.g. 6, 8, 10, 12, 14, 15) when water levels are high. It seems likely that the association of elevated sulphur with high water levels at some locations is due to the oxidation of sulphides in the upper aerobic part of the soil in the summer releasing soluble sulphates, which are then picked up by rising water levels in the winter. These observations have some potentially important implications for the management of the nationally important fen meadow and tall-herb fen communities for which Wicken Fen in famous.
The monthly monitoring of dipwell groundwater chemistry across Wicken Fen for the year April 2010–April 2011 brought to light considerable spatial and temporal variability, and highlighted the heterogeneous behaviour of major solutes (Ca, Na, Mg, S), nutrients (N, P, K) and trace elements (Al, Fe, Mn) across the site. From the analyses presented here it is evident that there is not a ‘one size fits all’ explanation for understanding the dynamics of groundwater and soil chemistry across the whole of Wicken Fen. Although it is very tempting to talk in generalities such as ‘Verrall’s Fen is more acid than Sedge Fen’, the tremendous variability between dipwells on Verrall’s Fen hints at a level and scale of complexity that a simple west-east dipwell transect can never hope to resolve. Repeating this work at a finer scale across the whole fen for several years would clearly help to resolve these complex relationships. Understanding the hydrology of each compartment of the fen, and the effect that groundwater has on soil chemistry and thus vegetation at the site would seem to be a sensible objective for long-term management.
Monitoring of more sites away from the two transects would help fill in the ‘gaps’ in knowledge about how representative the patterns observed here really are. Monitoring over several years would answer questions about long-term trends and possible cyclical patterns, whilst detailed studies of fen soil profiles might shed more light on the flux of major ions and nutrients in each season.
Ultimately, knowledge of how a system works is vital for the successful management of that system, and for spotting long-term trends that might be deleterious to that system if left unchecked. Understanding how Wicken Fen ‘works’ through time and in every dimension from the water supply to the soil, the vegetation and fauna, from the microscopic to the large scale, and from the physical to the chemical should be the eventual goal of any management strategy. Since resources are always limited, attention must be clearly focused towards those areas where problems have been identified, or where problems might arise. In this century, soil and water analyses should simply be tools, which stand alongside mowing regimes, grazing plans and scrub clearance in the fen manager’s armoury of appropriate technology to tackle whatever challenges the future may bring to Wicken Fen.
References
Ander EL, Shand P, Griffiths KJ, Lawrence A, Hart P, Pawley J (2004) Baseline Report Series: 13. The Great Ouse Chalk aquifer, East Anglia. Environment Agency
Anthony S, Duethmann D, Turner T, Carvalho L, Spears B (2008) Identifying the gap to meet the water framework directive—lakes baseline. Project WT0750C. DEFRA
Boreham S (2011) Geochemistry of water and soils in the area surrounding Dipwell 17 (Compartment 19) at Wicken Fen, Cambridgeshire. Unpublished Report for the National Trust
Boreham S (2013) Geology of the Wicken Vision Area, lower Cam valley, Cambridgeshire, UK. Neth J Geosci 92(1):47–59
Brightman MA, Bath AH, Cave MR, Darling WG (1985) Pore fluids from the argillaceous rocks of the Harwell region. Report of the Fluid Processes Research Group Institute of Geological Sciences, Flupu 85–86
Cook A, Harding M (2005) An evapotranspiration-groundwater balance for Wicken Fen. Ecology, Land and People, Report for The National Trust, Wicken Fen
Forster A, Hobbs PRN, Cripps AC, Entwisle DC, Fenwick SMM, Raines MR, Hallam JR, Jones LD, Self SJ, Meakin JL (1995) Engineering geology of British rocks and soils: Gault Clay. British Geological Survey Technical Report
Grieve IC, Gilvear DS, Bryant R (1995) Hydrochemical and water source variations across a floodplain mire, Insh Marshes, Scotland. Hydrol Process 9:99–110
McCartney MP, de la Hera A (2004) Hydrological assessment for wetland conservation at Wicken Fen. Wetl Ecol Manag 12:189–204
McCartney MP, de la Hera A, Acreman MC, Mountford O (2001) An investigation of the water budget at Wicken Fen. CEH Wallingford, Wallingford
Mountford JO, Stroh PA, Boreham S, Selby J, Sparks TH (2011) The vegetation of Wicken Fen NNR (Sedge and Verrall’s Fens): Preliminary Report on Results of the 2010 Survey. Unpublished Report for the National Trust
Petch JR (1977) Nitrates in surface waters of the Rother Basin, West Sussex. Unpublished PhD thesis King’s College, University of London
Shand P, Cobbing J, Tyler-Whittle R, Tooth A, Lancaster A (2003) Baseline Report Series: 9. The Lower Greensand of southern England. Environment Agency
Rowell TA (1997) The history of the fen. In: Friday LE (ed) Wicken Fen: the making of a Wetland Nature Reserve. National Trust and Harley Books, Colchester
Acknowledgements
The National Trust provided funding for the investigations in this study. The author would like to thank James Selby, John Hughes, Martin Lester and other NT staff for their help with collecting water samples and water level data during the study period.
Funding
This project did not have grant, but was commissioned and financially supported by the National Trust as a piece of baseline research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Boreham, S. Variations in groundwater chemistry and hydrology at Wicken Fen, Cambridgeshire, UK. Wetlands Ecol Manage 26, 17–35 (2018). https://doi.org/10.1007/s11273-017-9550-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-017-9550-2