Abstract
Constructed wetlands and constructed floating wetlands are widely used for nitrogen (N) removal from surface water to combat eutrophication in freshwaters. Two main N removal pathways in freshwaters are plant biomass N uptake and denitrification, i.e. transformation of nitrate (NO3-) to nitrous oxide (N2O) or nitrogen gas (N2) by different microbes possessing nirK, nirS, nosZI, and nosZII genes. In this study, we tested woodchips-based floating beds (WFBs) as a nature-based and environment-friendly method to remove nitrate-nitrogen (NO3-N) from water. Moreover, we tested whether WFBs could support the growth of three selected plant species and the abundance of microbes on plant roots and woodchips as a proxy for WFBs’ denitrification potential. We conducted a greenhouse experiment for 90 days and measured NO3-N removal rates from water in WFBs mesocosms during five sampling occasions. Plant biomass production, biomass N uptake, and plant morphology related to N uptake and abundance of denitrifying organisms were measured at the end of the experiment. NO3-N removal rates were 29.17 ± 11.07, 28.18 ± 12.62, 25.28 ± 9.90, and 22.16 ± 7.79 mg L–1 d–1 m–2 (mean ± standard deviation) in Glyceria maxima, Juncus effusus, Filipendula ulmaria, and unplanted WFBs treatments, respectively for whole experimental period. N content in above- and belowground biomass of studied species ranged between 0.98 – 1.15 and 1.09 – 1.28 (% dry weight), respectively. Plant relative biomass production was 215 ± 61, 67 ± 18, and 7 ± 17 (% dry weight) for G. maxima, J. effusus and F. ulmaria, respectively. Denitrifiers were detected both on plant roots and woodchips, indicating WFBs’ denitrification potential. Our study highlights that WFBs could be applied to enhance NO3-N removal from surface water through plant biomass uptake and denitrification processes. Future studies should consider the long-term in situ application of WFBs for NO3-N removal from water.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nitrogen (N) is one of the major elements causing loss of biodiversity in freshwater and other marine systems through eutrophication and has become a significant concern for human water security (Tickner et al., 2020; Glibert, 2017; Vörösmarty et al., 2010; Foley et al., 2005). Semi-natural and constructed wetlands (CWs) vegetated with aquatic plants have been applied in many parts of the world for nutrient retention, especially nitrogen (N) and phosphorus (P), and pollution control in aquatic systems (reviewed by Vymazal, 2011; Vymazal, 2007; reviewed by Wang et al., 2018). In recent years the concept of floating treatment wetlands has been developed and termed differently in literature such as constructed floating wetlands (Karstens et al., 2021), artificial floating beds (Bai & Xuan, 2022), artificial floating islands (Kong et al., 2019) or ecological floating beds (Wang et al., 2020a, b). This method has been tested in different freshwater systems including artificial eco-tanks, stormwater reservoirs, ponds, and lakes (Bi et al., 2019; Choudhury et al., 2019a; Shen et al., 2021). Previous studies applied floating beds for various purposes, such as improving the quality of mine process water (Choudhury et al., 2019a), agricultural and farming runoff (Spangler et al., 2019a), providing habitat for birds and aquatic organisms (Karstens et al., 2021) and, treating wastewater and sewage discharges (Afzal et al., 2019). One of the major advantages of floating bed treatment is that it can be applied in a land-based system for onsite treatment of highly nutrient-enriched water (e.g. up to 20 mg NO3-N L−1) for example, mine effluent (Choudhury et al., 2019a) and intensive recirculating aquaculture systems (Lindholm-Lehto et al., 2021) as well as in wetlands whose size are insufficient to remove a significant amount of nutrients.
Floating beds generally consist of a buoyant mat or structure, usually made of artificial plastic materials such as polyethylene, polypropylene, polyurethane, or polyvinyl alcohol foam (Karstens et al., 2021; reviewed by Wang et al., 2020a, b), floating in the water body and planted with emergent aquatic plants (Shen et al., 2021). Aquatic plants play a central role in the functioning of floating beds since they develop root systems in the water column as well as photosynthetic biomass above the floating beds (Pavlineri et al., 2017). The root systems developed beneath the floating beds take up nutrients from water and provide surface area for denitrifiers and could act as a hotspot for denitrification in the water column (Schwammberger et al., 2019). The major pathways of N transformation in floating beds are i) N assimilative uptake by aquatic plants (Keizer-Vlek et al., 2014; Schwammberger et al., 2019) and, ii) denitrification, conversion of nitrate to gaseous nitrogen compound performed by bacteria and archaea, that inhabit the plant root systems (Choudhury et al., 2019a; Zhang et al., 2024). The abundance of denitrifying genes (e.g. nirS, nirK, nosZI and nosZII) can be used as a proxy for the denitrification potential of different substrates such as plant roots and wetland sediment since a positive correlation between potential denitrification activities and the abundances of different denitrification genes have been shown by previous studies (Choudhury et al., 2022; Hallin et al., 2015; Ruiz-Rueda et al., 2009).
Artificial floating beds, however, have certain disadvantages, such as low nutrient removal efficiency and microbial habitat only fixed to plant roots that hinder the large-scale application of this method (Zhang et al., 2024). Another drawback of using floating beds made of artificial materials such as plastic is that plastics get eroded or degraded which adds microplastics to aquatic habitats and creates other environmental issues such as bioaccumulation and transformation in the food chain as well as ecotoxicological effects on aquatic organisms (Tang et al., 2021; Vo & Pham, 2021). This highlights the importance of developing innovative, cost-efficient and environmentally friendly variants of floating beds for long-term in situ applications. Hence, modified versions of floating beds such as hanging biofilm carriers below the floating beds (Messer et al., 2022; Sun et al., 2019) and using dried reed stems (Phragmites australis) instead of artificial plastics to support plant growth (Karstens et al., 2021) have been tested recently to increase the applicability of this method. In the current study, we propose the construction of floating beds with more environment-friendly materials that can support aquatic plant growth and enhance denitrification activities thus enhancing the overall N removal potential of floating bed treatment. We tested a new variant of floating beds made of woodchips packed in jute bags that would minimize the use of artificial polymers for constructing the main component of floating beds. The advantages of using woodchips are: i) they can be easily obtained from the local forest industry as a cheap forest by-product, ii) woodchips can be used as substrates for planting macrophytes in floating beds, iii) woodchips provide labile organic carbon and vast specific surface area for the growth of denitrifying bacteria (Goeller et al., 2019). Woodchips obtained from birch, pine, willow, and composite of hardwoods are commonly used in land-based woodchips bioreactors treating nutrient-rich water (Hellman et al., 2021; Jéglot et al., 2021; Kiani et al., 2022). However, to our knowledge, there is no available study directly investigating the suitability of woodchips-based floating beds (WFBs) for nitrate-nitrogen (NO3-N) removal from surface water.
We conducted a batch mesocosm experiment to assess the NO3-N removal from water by WFBs either planted with monocultures of three selected plant species or without any plant species as well as controls without any WFBs and plants. The objectives were to evaluate: i) WFBs’ effect on NO3-N removal, ii) species-specific performance on different parameters, i.e. plant establishment on WFBs, plant biomass production and biomass N accumulation, and abundance of denitrifying genes on roots and woodchips that was used as a proxy for denitrification potential of WFBs. We hypothesize that the addition of WFBs in mesocosms increases NO3-N removal from water compared to the mesocosms without WFBs. Our second hypothesis is that N removal from water, plant biomass production, N accumulation in plant biomass, and denitrifying gene abundances on plant roots differ among the studied plant species. Finally, we hypothesize that vegetated WFBs have higher N removal from water as well as higher abundance of denitrifying genes on woodchips compared to unvegetated WFBs.
2 Materials and Methods
2.1 Macrophytes Collection
We studied three common wetland plant species collected from wetlands near Kalmar, Sweden (56°40.110'N 16°17.745'E): Glyceria maxima (Hartm.) Holmb., Juncus effusus L. and Filipendula ulmaria (L.) Maxim. F. ulmaria is not a strictly aquatic plant but shows growth potential on floating bed treatment used for N uptake from water (Choudhury et al., 2019a). Specimens were collected in June 2020 and cultivated hydroponically in a greenhouse in plastic containers filled with tap water prior to the start of the experiment.
2.2 Construction of Woodchips Based Floating Beds (WFBs)
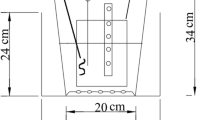
WFBs were made with woodchips, jute sheets and jute rope. Freshly produced woodchips (5 – 45 mm in size, without bark) from birch (Betula spp.) were collected from a local forestry industry. Jute bags were made with jute sheets and rope (Jula AB, Art. nr: 709017 & 388961) at a size of 50 × 30 × 10 cm (L × W × H) and then filled with 13 L (0.013m3) of fresh woodchips (ca. 4.5 kg) (see Fig. 1). The thickness of floating beds (or, planting substrate) may vary between commercial prototypes and experimental variants made of available materials. Commercial prototypes, for example, Beemats (New Smyrna Beach, FL, USA) has a thickness of 1 to 2 cm (Vo et al., 2023) Biohaven®, (Floating Island International, Shepherd, MT) has a thickness of 15 cm (Schwammberger et al., 2019) while Veg Tech floating island (Sweden) has a thickness 20 cm (Choudhury et al., 2019a). The bed thickness in experimental variants can be found in a range of 3 – 12 cm (reviewed by Arivukkarasu & Sathyanathan, 2023; Olguín et al., 2017; Yadav et al., 2023). Therefore, we expect that the woodchips thickness selected in this study should be sufficient to provide structural support and growth of plant roots. Before the main experiment, the WFBs were submerged in plastic containers filled with demineralized water for two weeks to make the woodchips softer to facilitate macrophyte growth.
2.3 NO3-N Removal and Plant Growth Experiment
The batch mesocosm experiment was conducted for 90 days in a greenhouse situated at Linnaeus University, Kalmar, Sweden. Prior to the experiment started, plastic container mesocosms (L × B × H: 58 × 39 × 30 cm, volume: 44L) (Jula AB, Sweden, art. nr: 004039) were filled with demineralized water for one week to allow potential contaminants to leach from the plastic. The water was then discarded. At the beginning of the N removal experiment, each mesocosm was filled with 40 L of nutrient-amended water free from N and P prepared with demineralized water according to Smart and Barko (1985). Subsequently, KNO3 and KH2PO4 were added to the water in each mesocosm to achieve the target concentrations of 20 mg NO3-N L−1 and 0.07 mg PO4-P L−1, respectively. Such nitrate levels in water can be found in the recipient of mine effluent (Choudhury et al., 2019a) and intensive recirculating aquaculture systems (Pedersen et al., 2012). Moreover, previous studies showed no negative effect of such levels of nitrate in water on the growth of macrophytes (Choudhury et al., 2018, 2019a, 2022). To reduce the algae growth in mesocosms the outer section of the plastic walls was wrapped with black plastic sheets.
We applied five types of treatments in the mesocosms: 1) WFB planted with G. maxima; 2) WFB planted with J. effusus; 3) WFB planted with F. ulmaria; 4) WFB without any plant species and, 5) mesocosms without any WFB and plant species (i.e. only 40 L nutrient amended water). Each treatment was replicated four times resulting in a total of 20 individual mesocosms (see Table S1, supporting information) and the mesocosms were randomly distributed in the greenhouse. The greenhouse was well equipped with plant growth lights and the day-night regime was set to 16:8 h. It is assumed that each mesocosm received equal amount of light during the experiment. In treatments 1 to 4, one WFB was installed in each mesocosm and WFB was submerged just below the water surface. Due to morphological differences, different planting approaches were adopted for different species. For example, in the case of G. maxima, six individual plants were planted in each WFB, while for J. effusus and F. ulmaria, six individual clumps were planted, respectively.
The initial total fresh weight of macrophyte biomass in each planted mesocosm was recorded. The dry weight of initial plant biomass was calculated based on the fresh weight – dry weight relationship measured for separate plant specimens for each species (Table S2, supporting information).
Our preliminary study showed that all NO3-N added in the planted and unplanted WFB mesocosms, except treatment no. 5, were completely removed from the water within 10 days after the addition. Therefore, we added nutrients from the stock solution in the mesocosms every second week to obtain a concentration of 20 mg NO3-N L−1 and 0.07 mg PO4-P L−1 as described above. Nutrient addition was made on a total of five occasions during the experiment (Table S3, supporting information). It should be noted the water in the mesocosms was not emptied during the experiment to avoid any kind of stress on plant growth. Nutrient addition, water sampling date, and treatment days on different occasions are given in Table S3 (supporting information). Before water sampling for NO3-N analysis, each mesocosm was filled with demineralized water to maintain a constant water volume of 40 L and to avoid the effects of water evaporation on water NO3-N concentration. NO3-N analysis was performed in the accredited laboratory ALS Scandinavia AB Luleå, Sweden according to the method CZ_SOP_D06_ 02_019 by discrete spectrophotometry with a detection limit of 0.06 mg NO3-N L–1. The water sample volume (50 mL) taken for the analyzing NO3-N was very small compared to the total volume of water (40L) in the mesocosm. Therefore, it is considered that the impact of sampling on the changes of the concentration of nitrate in the mesocosms was negligible. The NO3-N removal rate for each sampling occasion was calculated as (initial NO3-N concentration – end NO3-N concentration) / treatment days and expressed per unit area of WFB (mg NO3-N L–1 d–1 m–2). For treatment no. 5, the removal rate was expressed per unit surface area of the mesocosm instead of WFB.
At the end of the experiment, macrophytes biomass was harvested from each WFB, separated into aerial shoot biomass (termed as aboveground biomass, used throughout) and root plus biomass inside the WFBs (termed as belowground biomass, used throughout), cleaned with tap water to get rid of wood particles and other dead materials and dried at 60 °C for 7 days for dry weight measurement.
2.3.1 Nitrogen Content in Plant Biomass
Total-Nitrogen (total-N) content in harvested plant biomass was measured for each species on each WFB. The dried above- and belowground biomass samples were ground to a homogenous powder and weighed in tin capsules using a calibrated Satorius analytical balance (with an accuracy of four digit balance). A Thermo Scientific FlashEA 1112 Elemental Analyzer was used to analyze total-N. We calculated net plant N accumulation (g N m–2 of WFB) as defined as the standing stock (Richardson & Vymazal, 2001) of N content in above- and belowground biomass of each species per unit area (m2) of the WFB at the end of 90 days experimental growth.
2.3.2 Plant Morphological Study
We measured total (combined above- and belowground biomass) relative biomass production (%), final aboveground biomass (g – dry weight), final belowground biomass (g – dry weight), maximum root length (cm), and specific root surface area (cm2 g–1 dry weight– root) for each species at the end of the experiment.
The total relative biomass production (%) for each species in each mesocosm was calculated as:
- W1:
-
initial total dry weight,
- W2:
-
end total dry weight
We randomly selected three individual plants for each species at the end of the experiment to measure root surface area. Root specimens were collected from all representative parts of the plant root system. The root biomass was gently cleaned with demineralized water and the washed root samples were then photographed with a Canon digital camera (model: EOS1300D). The images were analyzed for the measurement of root surface area with image analysis software called ImageJ (freely available at https://imagej.nih.gov/ij/ download.html). After photographing, the root samples were dried at 60 ℃ for 7 days to measure dry weight.
The biomass production provides information regarding the N uptake potential of a species (Cornelissen et al., 2003), while root information will provide indication of both denitrification and N uptake potential of a species in the water column (Cornelissen et al., 2003; Farrar et al., 2003; Hodge et al., 2009). For example, higher root biomass indicates a high surface area for denitrification bacteria to be colonized on, which in turn should increase the denitrification potential of macrophytes in WFBs. On the other hand, higher root biomass indicates higher nutrient absorption area thus higher nutrient uptake potential of a plant species.
2.4 DNA Extraction and Quantification of Denitrifying Genes
At the end of the experiment, subsamples of fresh woodchips and plant roots for each species from each WFB were collected to quantify denitrifying gene abundances. For each plant species, representative subsamples were collected from the whole root system. Roots were then gently washed with tap water to get rid of woodchip particles, gently dried in a paper towel to get rid of excess water, and cut into small pieces, according to Hallin et al. (2015). Woodchips in each WFB were properly mixed, and a subsample was taken and gently dried with a paper towel to get rid of excess water. Both roots and woodchip samples were stored at –80 ℃ until the DNA extraction. DNA was extracted from 0.5 and 1 mg fresh roots and woodchip materials, respectively by using DNeasy® PowerWater® Kit (QIAGEN) according to the manufacturer's instructions. The extracted DNA was frozen and stored at − 80 °C until further analyses. The quantity and quality of the extracted DNA were determined using the spectrophotometer Infinite M200 (Tecan AG).
Real-time quantitative polymerase chain reaction (qPCR) assays were performed using a RotorGene® Q equipment (QIAGEN). The qPCR reactions were carried out in 10 μL reaction solutions containing 5 μL Maxima SYBR Green Master Mix (Thermo Fisher Scientific Inc.), with an optimized concentration of forward and reverse primers, 1 μL of template DNA, and sterile distilled water. The gene-specific primer sets, optimized primer concentrations, and thermal cycling conditions for each target gene are shown in Table S2 of Supporting information. Standard curves for each target gene were prepared from serially diluted stock solutions of target sequences (Eurofins MWG Operon). All qPCR measurements were performed in triplicate and the absence of contaminations was verified against negative controls.
The quantification data were analyzed with RotorGene Series Software v. 2.0.2 (QIAGEN) and LinRegPCR program v. 2020.0 (Ruijter et al., 2009). The gene abundances were calculated as a mean of fold differences between a sample and each tenfold standard dilution in respective standard as recommended by Ruijter et al. (2009). The abundance of each target gene was presented as gene copy numbers per gram of dry weight (copies g-DW−1) roots or woodchips. The qPCR was used to quantify the denitrifying genes nirS and nirK, coding for the two nitrite reductases, and nosZI and nosZII, coding for the nitrous oxide reductase from clade I and II, respectively (Table S4 of Supporting information).
3 Statistical Analyses
We analyzed the NO3-N removal rates at two stages. First, we compared removal rates during five sampling occasions between the treatments “mesocosms with unplanted WFBs” and “mesocosms without any WFBs” to assess whether woodchips have any effect on NO3-N removal. Secondly, we compared the mesocosms with monocultures of three selected plant species and unplanted WFBs to assess the species-specific difference in NO3-N removal rates. In both stages, we used mixed model analysis where species treatment and sampling occasions (see Table S3, supporting information for treatment details) were fitted as fixed factors, and individual mesocosms were fitted as a random factor. In addition, we re-run the analyses with treatment days as a covariate to test the potential influence of variation in treatment days on overall NO3-N removal rates. Tukey’s HSD was used for post-hoc pairwise comparison of N removal rates in each sampling occasion between different species treatments.
A multifactorial ANOVA was performed to determine the general trend in net total–N uptake in the above- and belowground biomass of the different species in the WFBs at the end of the experiment. One-way ANOVA was performed to investigate the difference among studied species on relative biomass production, final above- and belowground biomass, maximum root length, and specific root surface area. Two-way ANOVA was performed to test the effect of plant species and substrates, i.e. plant roots and woodchips, on the abundances of denitrifying genes nirS, nirK, nosZI, and nosZII. Tukey’s HSD was used for post-hoc pairwise comparison of different species. Independent sample t-tests were done to compare denitrifying gene abundance on woodchips in planted and unplanted WFBs.
In the case of NO3-N removal rates, specific root surface area, and denitrifying genes (i.e. nirK, nirS, nosZI, and nosZII), data were log-transformed while square root transformation was made for relative biomass production to meet the assumptions of parametric tests. The analyses were performed using the software IBM SPSS Statistics for Macintosh, Version 26.0.
4 Results and Discussion
4.1 N Removal Rates from Water
In support of our first hypothesis, we observed that adding WFBs in mesocosms significantly increased the NO3-N removal rates (Table S5, supporting information) and rates were consistently higher in the treatment “mesocosms with unplanted WFBs” compared to that of “mesocosms without any WFBs” during all five sampling occasions (Table S3, supporting information). Similarly, a bioreactor system filled with birch (Betula pendula) woodchips treating aquaculture effluent showed higher N removal rates compared to the control bioreactor without woodchips (Lindholm-Lehto et al., 2021). At initial stage, woodchips might release small amount of nitrate in mesocosms but the rate of nitrate release should diminish rapidly. For example, Homyak (2006) reported nitrate release of 0.02 mg N kg−1 woodchips d−1 after 7 days, and 0.008 mg N kg−1 woodchips day−1 after 29 days of incubation. In contrast, several studies reported that NO3-N could be immobilized by woodchips accounting for 1.0 – 6.9% removal of added NO3-N in the system (Greenan et al., 2009; Moorman et al., 2010).
The macrophyte species-specific performance on NO3-N removal rates, when grown on WFBs, during five sampling occasion is shown in Fig. 2. Our mixed model analysis showed a significant individual effect of sampling occasions and interaction effect of sampling occasion and plant species treatments on NO3-N removal rates in WFBs mesocosms (Table 1). Neither plant species treatment nor the covariate treatment-days had any significant individual effect on overall NO3-N removal rates. Irrespective of species treatment, NO3-N removal rates on sampling occasions 7 August and 19 August were significantly higher than sampling occasions on 7 September, 21 September and, 5 October. Temporal variations in N removal rates in freshwaters, e.g. wetlands and rivers dominated by macrophytes, were also observed in previous studies and found to be highest in August and lowest in October (Choudhury et al., 2018; Riis et al., 2020). In our experiment, unplanted WFBs showed significantly lower NO3-N removal rates compared to other species treatments on the sampling occasions on 7 August and 7 September (Fig. 2, Supporting information S3) (Tukey’s pairwise comparison p < 0.05). F. ulmaria showed significantly higher N removal rates compared to G. maxima on the sampling occasion on 21 September (Fig. 2, Supporting information S3). However, we did not observe any difference between the species treatments on the sampling occasions on August 19 and 5 October (Fig. 2, Supporting information S3). Temporal differences in N removal rates were also observed for wetland macrophytes in previous studies (Manolaki et al., 2020; Wang & Sample, 2014). In floating bed treatments, N uptake was higher for Pontederia cordata in August while J. effusus showed higher uptake in September (Chance et al., 2019). Wang and Sample (2014) reported that P. cordata planted in floating bed treatments showed higher N removal rates compared to Schoenoplectus tabernaemontani in July while S. tabernaemontani showed higher removal rates in September and October. No difference was observed between the two species in August. These temporal differences in nitrogen removal by different macrophyte species highlight the importance of maintaining species diversity in WFBs (Moi et al., 2021; Wang et al., 2020a, b) to optimize the nutrient removal function of wetlands.
Nitrate-nitrogen (NO3-N) removal rates (mg N L –1 d–1 m–2) (mean ± SE) in the water in five water sampling occasions (i.e. 7 August, 19 August, 7 September, 21 September and 5 October) in mesocosms installed with woodchips-based floating beds (WFBs) either planted with F. ulmaria, G. maxima, J. effusus or without any plant species
In contrast to our second hypothesis, the overall NO3-N removal rates for the whole experiment did not differ between the three selected plant species (Table 1). The mean NO3-N removal rates in different species treatments during the experiment at mesocosms level were 1.16 ± 0.44, 1.12 ± 0.50, 1.00 ± 0.39, and 0.88 ± 0.31 (mean ± standard deviation) g d–1 m–2 for G. maxima, J. effusus, F. ulmaria and unplanted WFBs, respectively. Chance et al. (2019) found higher N removal rates for P. cordata (2.93 g m–2 day –1) compared to J. effusus (0.59 g N m–2 day –1) for the whole experiment. In another floating bed treatment experiment, N removal rates for Thalia dealbata were significantly higher than Lythrum salicaria during 36 weeks of the experimental period (Ge et al., 2016).
The difference in NO3-N removal rates between planted and unplanted WFBs was insignificant as opposed to our third hypothesis. However, mean removal rates in all three plant species in our experiment had increased NO3-N removal rates compared to that of unplanted WFBs. The enhanced NO3-N removal in planted WFBs might be due to the plant N uptake and root-associated denitrification in the water column (Keizer-Vlek et al., 2014; Shen et al., 2021). Kiani et al. (2022) showed that a woodchips bioreactor vegetated with floating hook-moss (Warnstorfia fluitans) had higher NO3-N removal rates compared to woodchips bioreactor without moss. The N removal rates in the treatment of G. maxima and J. effusus in our experiment were higher than the mixture of Canna flaccida and J. effusus (0.128 to 0.214 g d–1 m–2) (White & Cousins, 2013) and Iris pseudacorus (0.277 g d–1 m–2) (Keizer-Vlek et al., 2014) grown in WFBs. Moreover, J. effusus in our experiment showed two times higher removal rates compared to the value reported for this species by Chance et al. (2019). The relatively higher N removal rates in our study compared to the above mentioned studies could partially be attributed to the type of substrate used for constructing floating beds. These studies used artificial materials such as PVC pipes, chicken wire, Styrofoam mats, solid-core foam mats and, plastic cups that do not support the denitrification process. On the other hand, woodchips enhance the denitrification process by providing a large surface area and labile carbon for denitrifying bacterial growth (Hellman et al., 2021), which in turn increase the overall NO3-N removal rates in our WFB mesocosms. Moreover, NO3-N removal rates in unplanted WFBs in our study are comparable to the removal rates found in woodchips-based bioreactors treating mine effluent (Kiani et al., 2022).
4.2 Plant Establishment and Net Nitrogen Accumulation by Plant Biomass in WFBs
All three macrophyte species planted in WFBs survived during the 90-day experimental period indicating that our experimental period was sufficient for plant establishment. A previous study found that 11 weeks were sufficient for the root growth and adaptation of two macrophyte species, Typha domingensis and Schoenoplectus californicus, in a floating structure (Rigotti et al., 2021). Several macrophyte species tend to establish in WFBs within 4 to 6 weeks after planting (Chance et al., 2019; Spangler et al., 2019b). Moreover, a 10 cm thickness of woodchip bed was sufficient to support plant growth as we observed both roots and shoot developments in all planted WFBs. A previous study including eight wetland macrophyte species showed that biomass of fibrous- and rhizomatic-roots was highest in shallow sediment layers (e.g. between 0 to 15 cm depths) (Cheng et al., 2009). More than half of the fine roots (root diameter < 2 mm) biomass, providing structural support and nutrient transport function to plants, can be found in 0 – 10 cm depth (Proctor & He, 2019).
Net total-N accumulation in plant biomass at the end of the experiment varied significantly between the three studied species that supports our second hypothesis (Table 2). In general, net total-N accumulation was highest for J. effusus and lowest for F. ulmaria (Table 2). Different N accumulation in plant biomass was also observed for a number of emergent macrophytes grown in artificial floating beds for treating different types of N-rich water such as mine effluent, stormwater, and agricultural runoff (Chance et al., 2019; Choudhury et al., 2019a; Spangler et al., 2019b; White, 2021). There were also significant differences in net total-N accumulation in above- and belowground biomass of the studied species in our study. Aboveground biomass showed higher total-N accumulation compared to that of belowground biomass at the end of the experiment. P. cordata and J. effusus also showed higher N accumulation in shoot biomass compared to root biomass after 20 weeks of experimental growth on floating beds (Chance et al., 2019) We also observed an interaction effect between plant species and plant tissue types (i.e. above- and belowground biomass) on N accumulation (Table 2). For example, net total-N accumulation in the aboveground biomass of J. effusus was higher than that of G. maxima and F. ulmaria, while net total-N accumulation in belowground biomass of J. effusus was lower than G. maxima and as equal to F. ulmaria (Table 2). F. ulmaria showed similar net total-N accumulation in both above- and belowground biomass. After 91 days of experimental growth on floating beds, Typha latifolia showed similar total-N accumulation in both above- and belowground biomass while I. pseudacorus had higher shoot N accumulation compared to that of root (Keizer-Vlek et al., 2014).
In line with our study, the difference in N content in plant biomass was also observed between I. pseudacorus, Carex spp., P. australis, T. latifolia and Lythrum salicaria grown on floating beds (reviewed by Alberto et al., 2021). N content in above- and belowground biomass for these species ranged between 0.68 – 2.10 (% dry weight) and 1.09 – 2.82 (% dry weight), respectively (Alberto et al., 2021), while N content in above- and belowground biomass of F. ulmaria, J, effusus and G. maxima in our study ranged between 0.98 – 1.15 (% dry weight) and 1.09 – 1.28 (% dry weight), respectively. Although belowground biomass showed higher N content (% dry weight) than that of aboveground biomass, bulk N uptake (g N m−2 of WFBs) for aboveground biomass was higher compared to that of root biomass (Table 2A). Therefore measuring both N content and bulk N accumulation in plant biomass is important for the correct choice of plant species to grow on WFBs.
Higher bulk N assimilation in aboveground biomass compared to belowground biomass indicates the potential of permanent removal of N from water through harvesting of vegetation in WFBs. If macrophyte biomass on WFBs is not harvested before plant senescence, there is a possible risk that the accumulated N will be released into the water column due to vegetative die-back (Kröger et al., 2007; Zhao et al., 2012). Several studies suggested macrophyte harvesting as a management practice to maintain the nutrient removal function of WFBs (Chance et al., 2019; Pavlineri et al., 2017; Wang et al., 2014). Macrophyte harvesting is recommended when biomass nutrient content is the highest during the growth season, for example Wang et al. (2014) suggested harvesting of P. cordata in July or August and S. tabernaemontani in October to maximize the nutrient removal through biomass harvesting. On the other hand, harvesting of J. effuses is recommended in September (Chance et al., 2019). Macrophyte harvesting choice can also be dependent on species growth characteristics; for example, J. effusus is an evergreen species and therefore, has the potential for nutrient accumulation throughout the year if not harvested (Chance et al., 2019). On the other hand, most of the shoots of G. maxima die-back in November (Westlake, 1966) and will release the nutrient into the water column in case biomass harvesting is not performed. Our study showed that 0.98 – 2.64 g N could potentially be removed from per unit area of WFBs through harvesting of aboveground biomass of studied species after 91 days of experimental growth. In contrast to our results, White and Cousins (2013) reported higher bulk N uptake in root biomass compared to shoot biomass for J. effusus and C. flaccida grown on constructed floating beds. Chang et al. (2012) found almost an equal amount of bulk N uptake in root and shoot biomass of J. effusus grown on floating beds. These contrasting N uptakes by different plant species highlight that species diversity may increase the overall plant N uptake (White, 2021), an important ecosystem process, in floating bed treatments.
4.3 Plant Morphological Study in WFBs
We investigated five important plant morphological characteristics related to plant N uptake and denitrification potential. We found a significant difference in relative biomass production between the studied species that supports our second hypothesis (Table 3). A significant difference in relative biomass production was also observed among three macrophyte species grown on floating beds in the rank of T. dealbata > C. indica > Lythrum salicaria (Ge et al., 2016). Mean total (combined above- and belowground biomass) relative biomass production in our study was highest for G. maxima followed by J. effusus (Table 3). F. ulmaria had the lowest relative biomass production compared to other species (Table 3). Our result highlights that G. maxima is able to establish itself in WFBs relatively quickly compared to the other two species.
The end aboveground biomass of J. effusus (0.23 kg – DW m–2) was significantly higher than G. maxima (0.16 kg – DW m–2) and F. ulmaria (0.10 kg – DW m–2). The mean standing aboveground biomass of the studied species in our experiment ranged within the values (0.04 – 0.30 kg – DW m–2) reported for five aquatic plants grown in floating beds in North Italy (reviewed by Alberto et al., 2021). The standing aboveground biomass of J. effusus found in our study is comparable with the biomass production of J. effusus (0.24 kg – DW m–2) grown in floating beds in a coastal lagoon in the Southern Baltic Sea (Karstens et al., 2021). However, the mean standing aboveground biomass of F. ulmaria was lower than previous values (0.249 – 0.968 kg-dry weight m–2) reported for this species grown in constructed floating beds for three growing seasons (Choudhury et al., 2019a). Therefore, J. effusus is more suitable to plant in WFBs, if the management focus is N removal by harvesting macrophyte biomass.
The end root biomass of different species did not differ in our experiment (Table 3). Spangler et al. (2019b) found no difference in root biomass among Panicum virgatum, Carex stricta, and Agrostis alba after 8 weeks of experimental growth on floating beds although total (combined root and shoot biomass) plant dry weight differed. In the case of root morphologies, we did observe a significant difference between the species after about 13 weeks of experimental growth. For example, the mean maximum root lengths of G. maxima (77.75 ± 5.43 cm) and J. effusus (75.50 ± 4.04 cm) were higher than that of F. ulmaria (25.00 ± 2.00 cm). Similarly, the mean specific root surface area of G. maxima (6307 ± 1059 cm2 g–DW–1) and J. effusus (4609 ± 585 cm2 g–DW–1) was significantly higher than that of F. ulmaria (3131 ± 535 cm2 g–DW–1). Roots lengths for five macrophyte species ranged between 15.12 to 24.39 cm after 8 weeks of experimental growth on floating beds (Spangler et al., 2019b) while Typha domingensis and Schoenoplectus californicus obtained root lengths of 23.28 and 14.35 cm, respectively after 11 weeks of experimental growth on floating beds (Rigotti et al., 2021). Maximum root length for Carex appressa was measured as 219 cm after 12 months of growth on floating beds (Schwammberger et al., 2020). This finding highlights that root length under floating beds tends to increase with time and might vary among different species. Our study shows that plants grown in WFBs have the potential to facilitate both nutrient uptake and denitrification in water columns more than half a meter. Moreover, per unit plant roots developed under the WFBs have the potential to provide as high as 6307 cm2 of surface attachment area for microbial growth, thus enhancing denitrification activity in the water column.
4.4 Denitrification Potential of WFBs
We quantified the abundances of denitrifying genes on plant roots and woodchips to estimate the denitrification potential of woodchips-based WFBs after 90 days of the experimental period. Microbial communities can be stabilized in wetland microcosms within 75 – 100 days while denitrifying bacteria take approximately 75 days for their development (Kumar et al., 2020; Wang et al., 2016 and references therein). Therefore, we expect that the denitrifying bacterial community in woodchip floating beds as well as in plant roots were well established at the end of our experiment. In general, we detected denitrifying genes i.e. nirS, nirK, nosZI, and nosZII on plant roots as well as on woodchips in all WFBs.
Previous studies showed a positive correlation between potential denitrification activity on plant roots and denitrifying gene abundances of nirS, nirK, and nosZI associated with plant roots in constructed wetlands (Hallin et al., 2015; Yao et al., 2023). The abundances of denitrifying genes were consistently higher on plant roots compared to that of woodchips for nirS, nirK, nosZI, and nosZII, respectively in support of our third hypothesis (Table 4; Fig. 3). The mean abundances of nirK, nirS, nosZI and nosZII on plant roots were 25.42 × 108, 16.79 × 108, 6.94 × 108 and 5.10 × 108 (gene copies per g-DW roots), respectively while on woodchips the abundances were 27.8 × 107, 8.11 × 107, 6.79 × 107 and 3.02 × 107 for nirK, nirS, nosZI and nosZII, respectively. We speculate that plant roots can provide a higher amount of labile organic carbon to microbes than woodchips, resulting in a higher abundance of denitrifying genes on root biomass compared to woodchips (Chen et al., 2014). This might also explain a higher N removal trend in planted WFBs compared to unplanted WFBs. In a previous column experiment treating nitrate-rich mine effluent with dried shoots of Carex rostrata, dried barley straw and, pine woodchips, Hellman et al. (2021) consistently found higher abundances of denitrifying genes (i.e. nirK, nirS, nosZI, and nosZII) on plant substrate compared to woodchips. Moreover, the bacterial community diversity (Shannon's diversity index) on plant substrates (e.g. dried shoots of C. rostrate and barley straw) was significantly higher than on woodchips (Hellman et al. 2021). Therefore, substrate types i.e. plant roots and woodchips of WFBs may have a varying effect on the denitrification potential of WFBs. Moreover, abundances of nirK, nirS, and nosZI genes on the roots of all three species in our experiment were much higher compared to the abundances of similar genes (6.20 × 108, 2.90 × 108 and 4.02 × 108 for nirK, nirS, and nosZ genes, respectively) quantified on the roots of Canna indica in WFBs (Zhang et al., 2018). In contrast to our second hypothesis, we did not observe any difference between plant species on denitrifying gene abundances in our experiment (Table 4; Fig. 3). However, there was an interaction effect of plant species and substrate types (i.e. woodchips and plant roots) on abundances of denitrifying genes nirK and nirS. In the case of nirK, J. effusus and G. maxima had higher gene abundances on roots compared to woodchips while F. ulmaria showed no difference between roots and woodchips (Fig. 3A). In the case of abundance of nirS on woodchips, there was no difference between the species, while J. effusus and G. maxima had higher nirS genes on roots compared to that of F. ulmaria (Fig. 3B). Although not significant, we found that denitrifying gene abundances on woodchips were higher in WFBs planted with F. ulmaria compared to J. effusus and G. maxima (Fig. 3 A-D). This can be attributed to the difference in root growth strategies of studied species. We observed that most of the root biomass for F. ulmaria developed in the jute bags entangled with the woodchips, while for the other two species roots mainly developed below the jute bags in the water column with relatively less contact with woodchips. Therefore, it can be assumed that the denitrifying genes were more evenly distributed on roots and woodchips in WFBs planted with F. ulmaria resulting in higher abundances of denitrifying genes on woodchips compared to J. effusus and G. maxima. Different root growth strategies as well as different root lengths of selected species highlight that enhancing species diversity should increase the overall N removal efficiency of floating beds. Besides denitrifying bacteria, woodchips can harbor a diverse community of microbes and fungi as shown in studies treating aquaculture effluents (Aalto et al., 2022) and agricultural drainage water (Jéglot et al., 2021) with woodchips bioreactors. Fungi can be pathogenic to their host plants and might affect the plant productivity negatively, especially in plant monocultures (Mommer et al., 2018). However, such interaction is not reported for plant species grown on woodchip substrate (Audet et al., 2021; Fatehi-Pouladi et al., 2020). Any detrimental microbial growth on woodchips and its subsequent effects on plant roots should be tested in future studies.
5 Final Remarks
Our study highlights that WFBs made of woodchips and jute sheets are feasible for cultivating emergent aquatic plants and enhancing nitrate removal from water through plant N uptake and associated denitrification as reflected by the abundances of nirS, nirK, nosZI, and nosZII genes on plant roots and woodchips. All three plant species tested in our experiment survived during the experimental period and showed growth potential, especially G. maxima and J. effusus. Our study showed that installing WFBs planted with studied species in water bodies makes it possible to facilitate denitrification in water columns up to 75 cm depth by creating large habitats for denitrifying microbes. Moreover, in our experiment, net total-N uptake, plant biomass production, and N removal rates from water by WFBs showed comparable results with other studies. It could be emphasized here that floating beads made of environment-friendly material such as woodchips and jute sheets have similar nitrate removal potential compared to other variants of floating beds that are made of artificial materials such as plastic. We have conducted our experiment for one growth season. If planted floating beds are operated for more than one season, biomass harvesting should be considered to reduce the risk of nutrient release into water through plant die-back. The difference in studied species on N uptake, plant characteristics related to N cycling, and higher abundances of denitrifying genes on roots compared to woodchips highlight that we should maintain species diversity in WFBs to improve overall N removal from water. Therefore, future studies should include multiple species combinations and investigate their effects on plant N assimilation, biomass accumulation, and denitrification processes. It should also be considered that WFBs could release dissolved organic carbon (DOC) at initial stage (McLaughlan & Al-Mashaqbeh, 2009) causing brownification in water column. This can temporarily hamper growth of submerged aquatic plants in wetlands (Choudhury et al., 2019b). Besides N removal from water, future studies should also investigate the cost-effectiveness and longevity of such treatment methods. Our study does not allow us to do a detailed cost analysis since the WFBs were constructed at the laboratory and the experiment was run at a university greenhouse facility with locally collected plant species. Considering only the expenses of construction materials, the cost for 1m2 of WFBs was around $37. Woodchips bioreactor treating agricultural drainage water estimated 1.66 cm annual loss of woodchips layer (Plauborg et al., 2023). Accordingly, our WFBs should last more than six years if no woodchips are added. Considering WFB’s minimum woodchips thickness of 3 cm, it is required to add 0.07 m3 of woodchips per m2 of WFB every 4th year. However, long-term in situ studies are necessary to make a realistic life cycle cost analysis (LCCA) of the WFB method. The potential removal of other elements such as P and heavy metals, as well as other ecosystem services related to WFBs should also be investigated in future studies.
Data Availability
Data will be published in digital repository, for example ResearchGate to make it publicly available after the manuscript has been accepted.
References
Aalto, S. L., Suurnäkki, S., von Ahnen, M., Tiirola, M., & Pedersen, P. B. (2022). Microbial communities in full-scale woodchip bioreactors treating aquaculture effluents. Journal of Environmental Management, 301, 113852.
Afzal, M., Arslan, M., Müller, J. A., Shabir, G., Islam, E., Tahseen, R., Anwar-ul-Haq, M., Hashmat, A. J., Iqbal, S., & Khan, Q. M. (2019). Floating treatment wetlands as a suitable option for large-scale wastewater treatment. Nature Sustainability, 2(9), 863–871.
Alberto, B., Stefano, B., & Maurizio, B. (2021). Plant species for floating treatment wetlands: A decade of experiments in North Italy. Science of the Total Environment, 751, 141666.
Arivukkarasu, D., & Sathyanathan, R. (2023). Floating wetland treatment an ecological approach for the treatment of water and wastewater – A review. Materials Today: Proceedings, 77, 176–181.
Audet, J., Jéglot, A., Elsgaard, L., Maagaard, A. L., Sørensen, S. R., Zak, D., & Hoffmann, C. C. (2021). Nitrogen removal and nitrous oxide emissions from woodchip bioreactors treating agricultural drainage waters. Ecological Engineering, 169, 106328.
Bai, Y., & Xuan, W. (2022). Drag force coefficient of the flexible vegetation root in an artificial floating bed channel. Ecological Engineering, 179, 106619.
Bi, R., Zhou, C. Y., Jia, Y. F., Wang, S. F., Li, P., Reichwaldt, E. S., & Liu, W. H. (2019). Giving waterbodies the treatment they need: A critical review of the application of constructed floating wetlands. Journal of Environmental Management, 238, 484–498.
Chance, L. M. G., Van Brunt, S. C., Majsztrik, J. C., & White, S. A. (2019). Short-and long-term dynamics of nutrient removal in floating treatment wetlands. Water Research, 159, 153–163.
Chang, N. B., Islam, M. K., & Wanielista, M. P. (2012). Floating wetland mesocosm assessment of nutrient removal to reduce ecotoxicity in stormwater ponds. International Journal of Environmental Science and Technology, 9, 453–462.
Chen, Y., Wen, Y., Zhou, Q., & Vymazal, J. (2014). Effects of plant biomass on denitrifying genes in subsurface-flow constructed wetlands. Bioresource Technology, 157, 341–345.
Cheng, X. Y., Chen, W. Y., Gu, B. H., Liu, X. C., Chen, F., Chen, Z. H., Zhou, X. Y., Li, Y. X., Huang, H., & Chen, Y. J. (2009). Morphology, ecology, and contaminant removal efficiency of eight wetland plants with differing root systems. Hydrobiologia, 623, 77–85.
Choudhury, M. I., Hallin, S., Ecke, F., Hubalek, V., Juhanson, J., Frainer, A., & McKie, B. G. (2022). Disentangling the roles of plant functional diversity and plaint traits in regulating plant nitrogen accumulation and denitrification in freshwaters. Functional Ecology, 36(4), 921–932.
Choudhury, M. I., McKie, B. G., Hallin, S., & Ecke, F. (2018). Mixtures of macrophyte growth forms promote nitrogen cycling in wetlands. Science of the Total Environment, 635, 1436–1443.
Choudhury, M. I., Segersten, J., Hellman, M., Mckie, B. G., Hallin, S., & Ecke, F. (2019a). Importance of plant species for nitrogen removal using constructed floating wetlands in a cold climate. Ecological Engineering, 138, 126–132.
Choudhury, M. I., Urrutia-Cordero, P., Zhang, H., Ekvall, M. K., Medeiros, L. R., & Hansson, L. A. (2019b). Charophytes collapse beyond a critical warming and brownification threshold in shallow lake systems. Science of the Total Environment, 661, 148–154.
Cornelissen, J. H. C., Lavorel, S., Garnier, E., Diaz, S., Buchmann, N., Gurvich, D. E., Reich, P. B., ter Steege, H., Morgan, H. D., van der Heijden, M. G. A., Pausas, J. G., & Poorter, H. (2003). A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany, 51(4), 335–380.
Farrar, J., Hawes, M., Jones, D., & Lindow, S. (2003). How roots control the flux of carbon to the rhizosphere. Ecology, 84(4), 827–837.
Fatehi-Pouladi, S., Anderson, B. C., Wootton, B., Wallace, S. J., Bissegger, S., Rozema, L., & Weber, K. P. (2020). Influence of plant species on microbial activity and denitrifier population development in vegetated denitrifying wood-chip bioreactors. Plants (Basel), 9(3), 289-.
Foley, J. A., DeFries, R., Asner, G. P., Barford, C., Bonan, G., Carpenter, S. R., Chapin, F. S., Coe, M. T., Daily, G. C., Gibbs, H. K., Helkowski, J. H., Holloway, T., Howard, E. A., Kucharik, C. J., Monfreda, C., Patz, J. A., Prentice, I. C., Ramankutty, N., & Snyder, P. K. (2005). Global consequences of land use. Science, 309(5734), 570–574.
Ge, Z., Feng, C., Wang, X., & Zhang, J. (2016). Seasonal applicability of three vegetation constructed floating treatment wetlands for nutrient removal and harvesting strategy in urban stormwater retention ponds. International Biodeterioration & Biodegradation, 112, 80–87.
Glibert, P. M. (2017). Eutrophication, harmful algae and biodiversity—Challenging paradigms in a world of complex nutrient changes. Marine Pollution Bulletin, 124(2), 591–606.
Goeller, B. C., Burbery, L. F., Febria, C. M., Collins, K. E., Burrows, N. J., Simon, K. S., Harding, J. S., & McIntosh, A. R. (2019). Capacity for bioreactors and riparian rehabilitation to enhance nitrate attenuation in agricultural streams. Ecological Engineering, 134, 65–77.
Greenan, C. M., Moorman, T. B., Parkin, T. B., Kaspar, T. C., & Jaynes, D. B. (2009). Denitrification in wood chip bioreactors at different water flows. Journal of Environmental Quality, 38(4), 1664–1671.
Hallin, S., Hellman, M., Choudhury, M. I., & Ecke, F. (2015). Relative importance of plant uptake and plant associated denitrification for removal of nitrogen from mine drainage in sub-arctic wetlands. Water Research, 85, 377–383.
Hellman, M., Hubalek, V., Juhanson, J., Almstrand, R., Peura, S., & Hallin, S. (2021). Substrate type determines microbial activity and community composition in bioreactors for nitrate removal by denitrification at low temperature. Science of the Total Environment, 755, 143023.
Hodge, A., Berta, G., Doussan, C., Merchan, F., & Crespi, M. (2009). Plant root growth, architecture and function. Plant and Soil, 321(1–2), 153–187.
Homyak, P. M. (2006). Nitrogen immobilization by woodchip application: Protecting water quality in a northern hardwood forest (Master's thesis, State University of New York, College of Environmental Science and Forestry, UMI Number: 1437419).
Jéglot, A., Audet, J., Sørensen, S. R., Schnorr, K., Plauborg, F., & Elsgaard, L. (2021). Microbiome structure and function in woodchip bioreactors for nitrate removal in agricultural drainage water. Frontiers in Microbiology, 12, 678448.
Karstens, S., Langer, M., Nyunoya, H., Caraite, I., Stybel, N., Razinkovas-Baziukas, A., & Bochert, R. (2021). Constructed floating wetlands made of natural materials as habitats in eutrophicated coastal lagoons in the Southern Baltic Sea. Journal of Coastal Conservation, 25, 44.
Keizer-Vlek, H. E., Verdonschot, P. F., Verdonschot, R. C., & Dekkers, D. (2014). The contribution of plant uptake to nutrient removal by floating treatment wetlands. Ecological Engineering, 73, 684–690.
Kiani, S., Lehosmaa, K., Kløve, B., & Ronkanen, A. K. (2022). Nitrogen removal of mine- influenced water in a hybrid bioreactor with floating hook-moss (Warnstorfia fluitans) in cold climate conditions. Ecological Engineering, 177, 106562.
Kong, L., Wang, L., Wang, Q., Mei, R., & Yang, Y. (2019). Study on new artificial floating island removing pollutants. Environmental Science and Pollution Research, 26(17), 17751–17761.
Kröger, R., Holland, M. M., Moore, M. T., & Cooper, C. M. (2007). Plant senescence: A mechanism for nutrient release in temperate agricultural wetlands. Environmental Pollution, 146(1), 114–119.
Kumar, S., Pratap, B., Dubey, D., & Dutta, V. (2020). Microbial communities in constructed wetland microcosms and their role in treatment of domestic wastewater. In R. Bharagava (Eds.), Emerging eco-friendly green technologies for wastewater treatment. Microorganisms for sustainability (Vol. 18). Springer.
Lindholm-Lehto, P. C., Pulkkinen, J. T., Kiuru, T., Koskela, J., & Vielma, J. (2021). Efficient water treatment achieved in recirculating aquaculture system using woodchip denitrification and slow sand filtration. Environmental Science and Pollution Research, 28, 65333–65348.
Manolaki, P., Mouridsen, M. B., Nielsen, E., Olesen, A., Jensen, S. M., Lauridsen, T. L., Baattrup-Pedersen, A., Sorrell, B. K., & Riis, T. (2020). A comparison of nutrient uptake efficiency and growth rate between different macrophyte growth forms. Journal of Environmental Management, 274, 111181.
McLaughlan, R. G., & Al-Mashaqbeh, O. (2009). Effect of media type and particle size on dissolved organic carbon release from woody filtration media. Bioresource Technology, 100(2), 1020–1023.
Messer, T. L., Miller, D. N., Little, H., & Oathout, K. (2022). Nitrate-N removal rate variabilities in floating treatment wetland mesocosms with diverse planting and carbon amendment designs. Ecological Engineering, 174, 106444.
Moi, D. A., Evangelista, H. B. A., Mormul, R. P., Evangelista, L. R., & Thomaz, S. M. (2021). Ecosystem multifunctionality and stability are enhanced by macrophyte richness in mesocosms. Aquatic Science, 83, 53.
Mommer, L., Cotton, T. E. A., Raaijmakers, J. M., Termorshuizen, A. J., Ruijven, J., Hendriks, M., Rijssel, S. Q., Mortel, J. E., Paauw, J. W., Schijlen, E. G. W. M., Smit-Tiekstra, A. E., Berendse, F., Kroon, H., & Dumbrell, A. J. (2018). Lost in diversity: The interactions between soil-borne fungi, biodiversity and plant productivity. The New Phytologist, 218(2), 542–553.
Moorman, T. B., Parkin, T. B., Kaspar, T. C., & Jaynes, D. B. (2010). Denitrification activity, wood loss, and N2O emissions over 9 years from a wood chip bioreactor. Ecological Engineering, 36(11), 1567–1574.
Olguín, E. J., Sánchez-Galván, G., Melo, F. J., Hernández, V. J., & González-Portela, R. E. (2017). Long-term assessment at field scale of Floating Treatment Wetlands for improvement of water quality and provision of ecosystem services in a eutrophic urban pond. Science of the Total Environment, 584, 561–571.
Pavlineri, N., Skoulikidis, N. T., & Tsihrintzis, V. A. (2017). Constructed Floating Wetlands: A review of research, design, operation and management aspects, and data meta-analysis. Chemical Engineering Journal, 308, 1120–1132.
Pedersen, L. F., Suhr, K. I., Dalsgaard, J., Pedersen, P. B., & Arvin, E. (2012). Effects of feed loading on nitrogen balances and fish performance in replicated recirculating aquaculture systems. Aquaculture, 338, 237–245.
Plauborg, F., Skjødt, M. H., Audet, J., Hoffmann, C. C., & Jacobsen, B. H. (2023). Cost effectiveness, nitrogen, and phosphorus removal in field-based woodchip bioreactors treating agricultural drainage water. Environmental Monitoring and Assessment, 195(7), 849–849.
Proctor, C., & He, Y. (2019). Quantifying wetland plant vertical root distribution for estimating the Interface with the anoxic zone. Plant and Soil, 440, 381–398.
Richardson, C. J., & Vymazal, J. (2001). Sampling macrophytes in wetlands. In R. B. Rader, D. P. Batzer, & S. A. Wissinger (Eds.), Bioassessment and management of North American freshwater wetlands (pp. 297–337). Wiley.
Rigotti, J. A., Paqualini, J. P., & Rodrigues, L. R. (2021). Root growth and nutrient removal of Typha domingensis and Schoenoplectus californicus over the period of plant establishment in a constructed floating wetland. Environmental Science and Pollution Research, 28, 8927–8935.
Riis, T., Tank, J. L., Reisinger, A. J., Aubenau, A., Roche, K. R., Levi, P. S., Baattrup-Pedersen, A., Alnoee, A. B., & Bolster, D. (2020). Riverine macrophytes control seasonal nutrient uptake via both physical and biological pathways. Freshwater Biology, 65(2), 178–192.
Ruijter, J. M., Ramakers, C., Hoogaars, W. M. H., Karlen, Y., Bakker, O., van den Hoff, M. J. B., & Moorman, A. F. M. (2009). Amplification efficiency: Linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Research, 37(6), e45.
Ruiz-Rueda, O., Hallin, S., & Baneras, L. (2009). Structure and function of denitrifying and nitrifying bacterial communities in relation to the plant species in a constructed wetland. Fems Microbiology Ecology, 67(2), 308–319.
Schwammberger, P. F., Lucke, T., Walker, C., & Trueman, S. J. (2019). Nutrient uptake by constructed floating wetland plants during the construction phase of an urban residential development. Science of the Total Environment, 677, 390–403.
Schwammberger, P. F., Yule, C. M., & Tindale, N. W. (2020). Rapid plant responses following relocation of a constructed floating wetland from a construction site into an urban stormwater retention pond. The Science of the Total Environment, 699, 134372–134372.
Shen, S., Li, X., & Lu, X. (2021). Recent developments and applications of floating treatment wetlands for treating different source waters: A review. Environmental Science and Pollution Research, 28(44), 62061–62084.
Smart, R. M., & Barko, J. W. (1985). Laboratory culture of submersed fresh-water macrophytes on natural sediments. Aquatic Botany, 21(3), 251–263.
Spangler, J. T., Sample, D. J., Fox, L. J., Owen, J. S., Jr., & White, S. A. (2019a). Floating treatment wetland aided nutrient removal from agricultural runoff using two wetland species. Ecological Engineering, 127, 468–479.
Spangler, J. T., Sample, D. J., Fox, L. J., Albano, J. P., & White, S. A. (2019b). Assessing nitrogen and phosphorus removal potential of five plant species in floating treatment wetlands receiving simulated nursery runoff. Environmental Science and Pollution Research International, 26(6), 5751–5768.
Sun, S., Liu, J., Zhang, M., & He, S. (2019). Simultaneous improving nitrogen removal and decreasing greenhouse gas emission with biofilm carriers addition in ecological floating bed. Bioresource Technology, 292, 121944.
Tang, Y., Liu, Y., Chen, Y., Zhang, W., Zhao, J., He, S., Yang, C., Zhang, T., Tang, C., Zhang, C., & Yang, Z. (2021). A review: Research progress on microplastic pollutants in aquatic environments. Science of the Total Environment, 766, 142572.
Tickner, D., Opperman, J. J., Abell, R., Acreman, M., Arthington, A. H., Bunn, S. E., ..., & Young, L. (2020). Bending the curve of global freshwater biodiversity loss: An emergency recovery plan. BioScience, 70(4), 330–342.
Vo, H. C., & Pham, M. H. (2021). Ecotoxicological effects of microplastics on aquatic organisms: A review. Environmental Science Pollution Research, 28, 44716–44725.
Vo, T.-K.-Q., Vo, T.-D.-H., Ntagia, E., Amulya, K., Nguyen, N.-K.-Q., Tran, P.-Y.-N., Ninh, N.-T.-T., Le, S.-L., Le, L.-T., Tran, C.-S., Ha, T.-L., Pham, M.-D.-T., Bui, X.-T., & Lens, P. N. L. (2023). Pilot and full scale applications of floating treatment wetlands for treating diffuse pollution. The Science of the Total Environment, 899, 165595–165595.
Vörösmarty, C. J., McIntyre, P. B., Gessner, M. O., Dudgeon, D., Prusevich, A., Green, P., Glidden, S., Bunn, S. E., Sullivan, C. A., Liermann, C. R., & Davies, P. M. (2010). Global threats to human water security and river biodiversity. Nature, 467(7315), 555–561.
Vymazal, J. (2007). Removal of nutrients in various types of constructed wetlands. Science of the Total Environment, 380(1–3), 48–65.
Vymazal, J. (2011). Constructed wetlands for wastewater treatment: Five decades of experience. Environmental Science & Technology, 45(1), 61–69.
Wang, C. Y., & Sample, D. J. (2014). Assessment of the nutrient removal effectiveness of floating treatment wetlands applied to urban retention ponds. Journal of Environmental Management, 137, 23–35.
Wang, C. Y., Sample, D. J., & Bell, C. (2014). Vegetation effects on floating treatment wetland nutrient removal and harvesting strategies in urban stormwater ponds. Science of the Total Environment, 499, 384–393.
Wang, M., Zhang, D., Dong, J., & Tan, S. K. (2018). Application of constructed wetlands for treating agricultural runoff and agro-industrial wastewater: A review. Hydrobiologia, 805, 1–31.
Wang, Q., Xie, H., Ngo, H. H., Guo, W., Zhang, J., Liu, C., Liang, S., Hu, Z., Yang, Z., & Zhao, C. (2016). Microbial abundance and community in subsurface flow constructed wetland microcosms: Role of plant presence. Environmental Science and Pollution Research International, 23(5), 4036–4045.
Wang, W. H., Wang, Y., Sun, L. Q., Zheng, Y. C., & Zhao, J. C. (2020a). Research and application status of ecological floating bed in eutrophic landscape water restoration. Science of the Total Environment, 704, 135434.
Wang, X., Luo, B., Wang, L., Zhao, Y., Wang, Q., Li, D., Gu, B., Min, Y., Chang, S. X., Ge, Y., & Chang, J. (2020b). Plant diversity improves the effluent quality and stability of floating constructed wetlands under increased ammonium/nitrate ratio in influent. Journal of Environmental Management, 266, 110607–110607.
Westlake, D. F. (1966). The biomass and productivity of glyceria maxima: I. Seasonal changes in biomass. The Journal of Ecology, 54(3), 745–753.
White, S. A. (2021). Plant nutrient uptake in full-scale floating treatment wetlands in a Florida stormwater pond: 2016–2020. Water, 13, 569.
White, S. A., & Cousins, M. M. (2013). Floating treatment wetland aided remediation of nitrogen and phosphorus from simulated stormwater runoff. Ecological Engineering, 61, 207–215.
Yadav, S., Kumar, J., Malyan, S. K., Singh, R., Singh, O., Goyal, V. C., Singh, J., & Negi, R. (2023). Evaluating pilot-scale floating wetland for municipal wastewater treatment using Canna indica and Phragmites australis as plant species. Sustainability, 15(18), 13601.
Yao, D., Dai, N., Hu, X., Cheng, C., Xie, H., Hu, Z., Liang, S., & Zhang, J. (2023). New insights into the effects of wetland plants on nitrogen removal pathways in constructed wetlands with low C/N ratio wastewater: Contribution of partial denitrification-anammox. Water Research, 243, 120277.
Zhang, F., Wang, J., Li, L., Shen, C., Zhang, S., Zhang, J., Liu, R., & Zhao, Y. (2024). Technologies for performance intensification of floating treatment wetland–An explicit and comprehensive review. Chemosphere, 384, 140727.
Zhang, L. L., Sun, Z. Z., Xie, J., Wu, J., & Cheng, S. P. (2018). Nutrient removal, biomass accumulation and nitrogen-transformation functional gene response to different nitrogen forms in enhanced floating treatment wetlands. Ecological Engineering, 112, 21–25.
Zhao, F., Xi, S., Yang, X., Yang, W., Li, J., Gu, B., & He, Z. (2012). Purifying eutrophic river waters with integrated floating island systems. Ecological Engineering, 40, 53–60.
Acknowledgements
We would like to thank Henrik Hallberg and Anders Månsson for helping with setting up the greenhouse for the experiment and Matyas Baan for helping with laboratory analysis. We also thank Keit Kill for DNA extraction and qPCR analyses. We thank the anonymous reviewers for their valuable comments on this and an earlier version of the manuscript.
Funding
Open access funding provided by Linnaeus University. This study was financed by Stiftelsen Oscar och Lili Lamms Minne (grant no. FO2019-0012), Linnaeus University and by the Estonian Research Council (grant no PSG714).
Author information
Authors and Affiliations
Contributions
Maidul Choudhury and Samuel Hylander planned the research design and experiment; Maidul Choudhury and Marc Hauber did the experiment, Mikk Espenberg did the microbial analyses, Maidul Choudhury did the statistical analysis and develop the manuscript with Samuel Hylander, Kuno Kasak, Mikk Espenberg and Marc Hauber. Funding acquisition by Maidul Choudhury, Samuel Hylander and Kuno Kasak. All authors critically commented and reviewed the final draft of the manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable for current study.
Consent to Participate
Not applicable for current study.
Consent to Publish
We affirm that this work has not been published in print or electronically, and is not under consideration for publication elsewhere. All authors agreed with the final content of the manuscript and all gave explicit consent to submit it to journal Water, Air, & Soil Pollution.
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Choudhury, M.I., Espenberg, M., Hauber, M.M. et al. Application of Floating Beds Constructed with Woodchips for Nitrate Removal and Plant Growth in Wetlands. Water Air Soil Pollut 235, 493 (2024). https://doi.org/10.1007/s11270-024-07275-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07275-2