Abstract
The study evaluated calcium-rich deinking paper sludge (DPS) biochar's capability as a viable alternative method to mitigate soil cadmium (Cd) availability. Our analysis of 68 recent studies showed that 75% of the studies focused on contamination levels below 10 mg kg-1. However, mining and smelting areas exhibit higher levels of Cd contamination (mean value of 57.5 mg kg-1 with a CV of 128%), necessitating a contamination rate-dependent approach.
Clay loam (CL) and sandy loam (SL) soils were artificially contaminated with Cd to mimic polluted areas (20, 40, 80 mg kg-1). Soils were aged for six months and then treated with DPS biochar doses of 0%, 1%, and 3% (w/w) for a month. Cd extractability and toxicity were gauged using diethylenetriaminepentaacetic acid extraction and plant physiology tests. Supplementarily, machine learning algorithms were tested to predict plant physiological parameters and biomass production, leveraging variables from principal component analysis and design parameters.
Biochar application (3%, w/w) reduced soil Cd availability (20.1% in SL, 8.4% in CL; p < .05), attributed to increased soil pH, enhanced microbial activity, and expanded soil surface area. The plants grown in treated soils displayed increased dry matter content, chlorophyll, relative water content, and decreased malondialdehyde levels. The impact varied, being more pronounced in SL soils with high Cd contamination.
This study presents the first report on the use of DPS biochar in Cd-contaminated soils and sets expectations for its outcomes regarding plant physiology and soil microbial activity in a diversified experimental design. DPS biochar appeared as a tool for mitigating soil Cd availability and alleviating plant stress particularly in SL soils. The biochar's efficiency was influenced by its dose, the level of contamination, and the soil type, highlighting the importance of tailored application strategies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Heavy metals, known for their persistence in soil over prolonged periods, can accumulate to toxic levels, presenting a significant risk to plants, animals, and humans (Bolan et al., 2014). Cadmium (Cd) is one such heavy metal frequently found in industrial and agricultural operations such as mining (Lu et al., 2019; Xie et al., 2023), smelting (Yang et al., 2022), and by use of phosphate fertilizers (Park et al., 2021).
While typical agricultural soils have Cd concentrations below 5 mg kg-1, extreme cases can exceed 100 mg kg-1 (Shi et al., 2019). According to Hu et al. (2020), as reported in their meta-analysis, remediation efforts in arable soils typically involve experiments with a Cd content range of 0 to 10 mg kg-1. In comparison, mining and industrial pollution result in higher averages, with some areas like Kütahya, Turkey, recording Cd levels exceeding 200 mg kg-1 (Arslan and Çelik, 2015; Palutoglu et al., 2018). Research from Northern Tunisia found a median of 19 mg kg-1 average Cd in top-soils affected by mining activities (Boussen et al., 2013). The textile industry and nonmetallic mineral production result in average Cd levels of 42 mg kg-1 and 25.8 mg kg-1, respectively (Kabir et al., 2012). In this context, although concerns about Cd contamination in farming primarily relate to crop uptake and food chain exposure, in severely contaminated areas such as mines and mine tailings, the primary exposure routes might be dust inhalation, direct soil ingestion, or water consumption from runoff. Each pathway carries distinct risks that require different mitigation strategies.
A meta-analysis of 68 studies from ScienceDirect (Jan 1, 2022 - June 10, 2023) showed that 75% of Cd remediation research using biochar addressed contamination levels of 10 mg kg-1 or less (Fig. 1a). Polluted arable soils contain about 2.78 mg kg-1 Cd, while artificially contaminated ones average 24.3 mg kg-1 (Fig. 1b). In contrast, soils affected by mining or smelting have much higher Cd levels, averaging 57.5 mg kg-1, which hinders crop cultivation. Moreover, even though existing literature suggests that the adsorption behavior and remediation efficiency of biochar fluctuates based on contamination levels (Dai et al., 2016; Kováčik et al., 2022), recent research on contaminated soils often focuses on a fixed level of contamination (Fig. 1c). To enhance predictions of biochar's impact, comprehensive environmental modeling is imperative, accommodating diverse contamination rates and specific biochar applications.
An analysis of recent Cd contamination studies. (a) Histogram illustrating the distribution of maximum Cd contamination levels reported in recent studies. (b) Categorization of the highest contamination levels investigated in recent studies, differentiated by the source of pollution: direct influence of mining and smelting (M./S.), artificial contamination (A. P.), and agricultural activity including the use of contaminated irrigation water (Ag. A.); whiskers represent mean ±1.5*standard deviation. (c) A representation of the availability of contamination level-based investigations in recent studies, derived from a search of the ScienceDirect database using the terms “soil”, “cadmium”, and “biochar” (studies that are available online from 01.2022 to 06.2023). Detailed information on the meta-analysis studies, including remediation mechanisms, details of biochar type and application rate, is provided in Table S1
In soils that are highly contaminated and unsuitable for crop production, it is prudent to consider feasible biochar solutions, such as from deinking paper sludge (DPS). This material is an environmentally benign by-product of the paper industry (Abida et al., 2023) with a rich carbonate and fibre content but limited plant nutrients (Calace et al., 2005). On average, dry sludge production amounts to between 40-50 kg per tonne during paper recycling (Bajpai, 2015; Singh et al., 2018). Specifically, DPS generation varies from 170-600 kg per tonne, depending on the type of paper, such as newsprint to tissue paper (Lou et al., 2012; Bajpai, 2015), leading to considerable solid waste accumulation. Early research has indicated that DPSs enhance agricultural soil conditions (Camberato et al., 2006; Ziadi et al., 2013) and have applications in bioremediation by immobilizing heavy metals (Calace et al., 2005; Battaglia et al., 2007; Bonoli & Dall’Ara, 2012). DPSs are also utilized in building materials and bioenergy (Vashistha et al., 2019; Chakraborty et al., 2019) and as soilless growth mediums (Vannucchi et al., 2021). Although biochar products derived from DPSs are less studied than their raw counterparts, they have found success in environmental applications such as removing copper (II) from polluted water (Méndez et al., 2009), and serving as calcium (Ca) rich adsorbents to filter phosphorus from eutrophic water (Wang et al., 2021). Over the years, there's been a growing interest in their soil amelioration properties. However, this interest remained limited to the remediation of Ni and Zn-polluted soils (Méndez et al., 2014; Paz-Ferreiro et al., 2017).
Different sludges like sewage (Sun et al., 2023), manure (Zhao and Naeth, 2022), and food waste-based (Tsadik et al., 2020) are used post-pyrolysis for remediation. While the effectiveness of biochar in immobilizing metals is influenced by its structure and surface traits (Zhou et al., 2018), the high fibre and carbonate content of biochar feedstocks, similar to DPS, is known to enhance functional group content during pyrolysis and improve pH buffering capabilities, respectively (Chin-Pampillo et al., 2021; El-Naggar et al., 2022). These properties are important for potential remediation efforts (Uchimiya et al., 2010; Phillips et al., 2016). In this context, recent research supports the benefits of Ca loaded and carbonate-rich biochars (Jalalabadi et al., 2019; Liu et al., 2021; Shen and Yuan, 2021). In Cd-polluted soils, the antagonistic relationship between Cd and Ca leads to improved plant metabolism due to the ionic mimicry (Kováčik et al., 2020). As a result, DPS biochar is emerging as a promising but scarcely researched alternative to other sludge-based biochars for reducing heavy metal availability.
The effectiveness of biochar across different contamination scenarios, especially its influence on soil microcosm activity, is crucial to understand (Qu et al., 2022). Biochars can decrease Cd bioavailability and lessen its toxicity to soil microbes, leading to a more vibrant microbial community and heightened enzymatic functions. They are also known to promote microbes that render Cd into less harmful formes (Xia et al., 2021). Acting in conjunction with its resident microorganisms, biochars can mitigate the availability of environmental pollutants through a variety of processes, including binding, sorption, precipitation, and microbial-led bioaccumulation or transformation (Blenis et al., 2023). Soil dehydrogenase activity (DHG), an indicator of microbial activity related to cellular respiration and energy production (Saha et al., 2012), can indicate diminished Cd toxicity post-biochar treatment, signifying increased microbial respiration (Wang et al., 2022). Furthermore, beta-glucosidase activity (BGLU), vital for the soil carbon cycle, can rise after biochar use (Lu et al., 2015), indicating enhanced functions of decomposition and nutrient cycling (Adetunji et al., 2017).
In regions with high Cd exposure, the impact on buffer crops' physiology holds significant relevance for sustainable remediation. Cd compromises plant health by affecting chloroplasts, decreasing chlorophyll, and thus hampering photosynthesis in species like mung bean (Wahid et al., 2008), wheat (Moussa and El-Gamal, 2010), and rapeseed (Baryla et al., 2001). Eventually, Cd triggers oxidative stress, which can be represented by elevated malondialdehyde (MDA) levels, which impede plant growth (Mulenga et al., 2020; Kessler et al., 2022). Integrating these productivity and stress indicators is essential for land management strategies aimed at counteracting Cd contamination while providing a better understanding of the efficacy of biochar applications.
The focus of the current study was to simulate highly contaminated areas affected by mining and industrial activities, which typically do not support food production and tolerate minimal nutrient input. Different doses of biochar were applied to artificially contaminated Entisols of two different textures, facilitating an evaluation of application and contamination rate dependent changes in Cd availability in relation to soil texture. Based on this approach, the objectives of this study were to highlight a viable, new way to reduce Cd availability in soils while offering a waste management strategy to the paper recycling industry. In connection with the aforementioned previous literature, the shifts in the soil microbial activity indicators were examined as well as growth of wheat plants in the treated soils; To the best of collective knowledge, this research represented the first study examining the effects of DPS biochar on both soil and plant health under conditions of elevated Cd pollution.
2 Materials and Methods
The research framework presented here focused respectively on the (i) production and characterization of DPS biochar, (ii) artificial soil contamination with Cd, (iii) aging of experimental soils with Cd, and (iv) subsequent incubation of different experimental soils with the produced biochar. After the incubation period, (v) changes in soil Cd availability and enzymatic activity were determined, followed by the growth of (vi) wheat seedlings in the experimental soils, and their physiological stress responses under Cd contamination. The analytical and statistical methods adopted throughout the experiment are described in the current section.
2.1 Production and Analysis of Deinking Paper Sludge Biochar
DPS material was provided, in an agreement, by a freelance entrepreneur requesting characterization of the material by the service procurement from Ege University Agricultural Faculty (EUAF). The biochar was produced using a muffle furnace (MT9507-P, Magmatherm Inc., Turkey) from DPS at 500 °C for 1 h, with a heating rate of 10 °C increase per minute, under limited oxygen conditions. The process took place in an enclosed steel container with a wall thickness of 1 cm and inner dimensions of 14 cm (W) x 14 cm (H) x 18 cm (D), aiming to implement a cost-effective production process.
The pH and the electrical conductivity (EC) of the biochar material were determined in a 1:5 dilution of distilled water using a WTW Inolab pH-meter 7110 and an EC-meter Cond 720 (Xylem Analytics Germany Sales GmbH & Co, Germany) (Singh et al., 2017). The total contents of heavy metals, micro- and macro-nutrients (except nitrogen) were determined according to Zarcinas et al. (1996), following the digestion of the biochar material in a HNO3:HCl (1:3) mixture. The extractable heavy metal and micronutrient contents were determined after extraction with diethylenetriaminepentaacetic acid (DTPA) + CaCl2 + triethanolamine solution (pH: 7.2) (Lindsay & Norvell, 1978). The heavy metal, micronutrient and Mg contents were measured using atomic absorption spectrophotometry (AAS, SpectrAA Fast Sequential Flame Model 240 FS, Varian Inc. USA), while the K, Ca, and Na were measured using a flame photometer (Eppendorf Inc., Germany). Total nitrogen was determined according to Bremner (1965) by a modified Kjeldahl method, and total phosphorus was determined colorimetrically (CamSpec M302, Camspec Ltd, United Kingdom) using the vanadomolybdophosphoric acid method (Enwezor, 1976). The hygroscopic moisture saturation was assessed by determining the water-holding ability of the biochar under enclosed conditions of 100% relative humidity, while the surface area was estimated by its water holding ability in 20% relative humidity using the following equation (Eq. 1; Sridharan and Rao 1972):
where S is the specific surface area in m2g-1, W is the equilibrium moisture content in g water adsorbed per g of biochar, N is Avogadro's number (6.025 x 1023), M is the molecular weight of water (18.016 g), and A is the area in square angstroms per water molecule (10.8 Å2).
The DPS biochar's ash and volatile matter contents were determined per a modified (525°C for ash, 600°C for volatile matter) D1762 method from the American Society for Testing and Materials International (ASTM International, 2007). The biochar's fixed carbon content was determined by accounting for ash and volatile matter, with its ash content falling between 88% (Méndez et al., 2014) and 93% (Paz-Ferreiro et al., 2017) as shown in Table 1. Both referenced studies, which used a nitrogen atmosphere in proximate analysis, and produced biochars at 500°C, reported higher fixed carbon contents. Importantly, the considerable degradation of calcium carbonate, along with associated mass losses, begins at temperatures above 600°C (Singh & Singh, 2007). The biochar had a mostly carbonate-associated Ca content exceeding 20%. This value is not only significant in distinguishing the product from the latter study mentioned but also aligns closely with another DPS material used in thermochemical conversion, as reported by Lou et al. (2012) with a content of 19%.
2.2 Preparation and Analysis of Experimental Soils
The sandy loam (SL) and clay loam (CL) soils used in this experiment were collected in Izmir, Turkey from the alluvial plains located at Menemen Research, Application and Production Farm of the Ege University Agricultural Faculty (EUAF) and the experimental olive garden of the EUAF Department of Soil Science and Plant Nutrition, respectively.
Each experimental soil was sieved through a 1 cm mesh, divided into three parts and each part was mixed with a solution of 3CdSO4 · 8H2O to achieve contamination levels of 20, 40, and 80 mg kg-1 Cd for different classes of soil texture. The contaminated soils were weighed in 5 kg pots and were left to age with Cd for 6 months, with the moisture level kept at 60% of the field capacity through capillary irrigation every other day. The aging process was applied after the artificial contamination to reduce the initial ionic strength of spiked Cd in the soil solution. This was done to effectively simulate polluted soils containing both residual and active forms of Cd. The soils were then air-dried and sieved again through a 1 cm mesh and were mixed with 1% or 3% (w/w) DPS biochar that had been sieved through a 1 mm mesh to increase its specific surface area. A total of 18 soil contamination scenarios were investigated (Table 2), with 5 replications for each experimental treatment. The soils mixed with biochar were left to incubate for one month under the same conditions as the Cd aging.
Soil pH and EC values were determined in saturated paste (Robbins & Meyer, 1990) using Inolab pH-meter 7110 and EC-meter Cond 720 (Xylem Analytics Germany Sales GmbH & Co., Germany) for characterization of the soils before the experimental phase and to investigate the changes post-incubation. The enzymatic activity of the soils was analysed post-incubation to assess the influence of biochar treatments on microbial activity. The extractable Cd contents were determined after DTPA extraction (Lindsay & Norvell, 1978) by atomic absorption spectroscopy (SpectrAA Fast Sequential Flame Model 240 FS). The dehydrogenase enzyme activity (DHG, EC 1.1.1) was determined using a modified method of Thalmann (1968). Briefly, soil samples were incubated on an orbital shaker with triphenyl tetrazolium chloride solution for 16 h at 25 °C. The resulting triphenyl formazan (TPF) product was extracted with acetone and was measured photometrically at 546 nm using a spectrometer (CamSpec M302, Camspec Ltd., United Kingdom). The activity of the enzyme beta-glucosidase (BGLU, EC 3.2.1.21) was evaluated using the method developed by Hoffmann & Dedeken (1965). Soil samples were incubated with the substrate β-glucosido-saligenin (salicin) for 3 h at 37°C. The amount of saligenin released from the substrate was then determined by a colorimetric method at 578 nm, using the spectrophotometer and a colouring agent of 2,6-dibromoquinone-4-chlorimide. Besides these analyses, initial selected properties of each experimental soil before Cd contamination were determined and reported in Table 3.
2.3 Plant Growth and Stress Parameters
Wheat seeds (Triticum aestivum L., cv. Cumhuriyet-75) were surface sterilized by soaking in 4% NaClO and 70% ethanol, respectively, for 1 min and washed 5 times in sterile distilled water. The seeds were then germinated in filter papers moistened with 1 mM of CaSO4 solution. Five days after germination, the seedlings were transplanted into polyethylene pots containing 250 g of soil that had undergone a 6-mo aging process followed by a 1-mo biochar incubation phase, and placed in a controlled growth chamber with a 16 h light/8 h dark cycle, a temperature of 22°C, light intensity of 100 μmol m-2 s-1, and relative humidity of 70%. To take advantage of the seedling stage's sensitivity towards heavy metal stress (Baruah et al., 2019) and rapidly assess manifested stress response under controlled plant growth conditions, we measured shoot dry matter content, relative water content (RWC), chlorophyll a and b content, ion leakage rate (IL), and MDA content in 21-day-old plants.
The dry matter content of the plants was determined as follows: The fresh biomass of the shoots (n=5) was weighed. Subsequently, the samples were dried at 70 °C for 72 hours, after which their dry biomass was weighed, and the percent dry matter content was determined according to Hunt et al. (2002).
The RWC was calculated as follows: Five leaves of approximately the same size were taken from the plants in each group, and their fresh weight was measured. The leaves were then immersed in deionized water for 6 h under low light to reach turgor. At the end of this period, turgor weight was determined. The leaf samples were then dried in an oven at 70 °C for 72 h and their dry weight was determined. The RWC of the leaf samples belonging to each group was calculated using Eq. 2 described by Schonfeld et al. (1988).
The contents of chlorophyll a and b were determined as follows: A 0.25 g sample was taken from the middle part of the leaves and was homogenized with 20 ml of 80% acetone. The homogenate was filtered through a black filter paper, and the filtrates were placed in spectro-cuvettes and were measured at wavelengths of 663 nm and 645 nm for chlorophyll a and b content. The values obtained were used in Eq. 3 and 4 to calculate the chlorophyll a and b content in mg l-1 (Arnon, 1949):
The IL rate was calculated by measuring the electrolyte emitted from the plant cells (Fan & Blake, 1994). Approximately 2 cm of leaf discs were placed in double distilled water for 5 h and EC value of the solution was measured (EC1). The same solution with the discs was then heated at 100°C for 10 min, and the EC value of the solution was measured again (EC2). The IL rate was calculated using the following equation (Eq. 5):
The amount of MDA, the product of lipid peroxidation, was estimated using the thiobarbituric acid reaction method (Rao & Sresty, 2000). Leaves (0.1 g) were homogenized in 3 ml of 0.05 M Tris-HCl buffer with pH 7.4. After homogenization and subsequent centrifugation (20 min at 13,200 rpm), thiobarbituric acid (0.5% [w/v] in 20% [w/v] trichloroacetic acid) was added to an aliquot of the supernatant and the mixture was then heated in a boiling water bath (within 90 °C) for 30 min. After cooling and centrifugation (20 min at 13,200 rpm), the absorbance of the supernatant was measured at 532 nm and was corrected for non-specific turbidity by subtracting the absorbance at 600 nm. A solution of 0.5% thiobarbituric acid [w/v] in 20% [w/v] trichloroacetic acid was used as a blank. The amount of MDA per g of fresh weight was calculated using the MDA extinction coefficient of 0.156 μM-1 cm-1.
2.4 Statistical Analyses
All statistical analyses and visualizations were performed using STATISTICA 13.5 software (TIBCO Statistica®, USA). To compare the changes in soil properties and the plant stress parameters under biochar application and Cd contamination conditions, one-way variance analysis (ANOVA) was performed and p-values below .05 were considered statistically significant (Duncan’s test). To identify correlations between plant stress parameters and biochar application rate or Cd contamination rate, Pearson’s correlation coefficient was used and p-value below .05 was reported with precision. To illustrate the differences based on the biochar application rate and the Cd contamination rate, contour graphs of IL rate and MDA were plotted using Wafer fit with embedded mean values of the homogeneous groups revealed by ANOVA.
A supplementary machine learning investigation was conducted on plant physiological parameters, with the aim of estimating one from another, predictions on biomass production and stress responses. Previous studies have looked at prediction of biochar properties with different MLAs regarding the adsorption of heavy metals in aqueous environments (Wong et al., 2020) and in soil (Palansooriya et al., 2022). However, the current scientific literature reveals a notable gap in studies focusing on the prediction of plant physiological responses or biomass production. Initially, the high weights of parameters such as the IL rate and RWC, as presented in the principal component analysis, were taken into account. Their capability to explain the variance within the dataset (Fig. S1) was harnessed within a general regression tree model. Alongside the design parameters of the study, which included the biochar application rate, contamination level, and soil type, these variables were used to estimate the chlorophyll a content, MDA, the dry matter content, the dry biomass, and the fresh biomass of the plant materials. The initial estimations, combined with the actual IL rate, RWC values, and design parameters, were again integrated within various machine learning models to enhance prediction accuracy. The models utilized in this supplementary work were general regression/classification trees (GCRT), automated neural networks (ANN), support vector machines (SVM) and multivariate adaptive regression splines (MARS). Each model's performance was evaluated by assessing the coefficient of determination (R2) and the root mean square deviation values between observations and predictions. Detailed model specifications and variables are presented in Tables S2 and S3, respectively.
3 Results
3.1 Changes in Soil Properties
This study revealed that the soils contaminated with Cd at concentrations of 40 and 80 mg kg-1, but not treated with biochar, exhibit significant reductions in DTPA extractable Cd concentrations from the initially added amounts following a 7-mo period (Table 4). This reduction was more pronounced in the case of CL soils. After the aging period, the DTPA-extractable Cd content remained at 74% of the initial amount in SL40 soil, compared to 64% in CL40 soil. In soils contaminated with 80 mg kg-1 of Cd, the reduction in Cd availability was slightly higher. Specifically, of the added Cd, only 54% remained DTPA-extractable in CL80 soil, compared to 59% in SL80 soil.
DPS biochar was hypothesized to enhance the reduction of Cd bioavailability in soil. To test this, the impact of biochar applications on the DTPA-extractable Cd content of both CL and SL soils was separately evaluated for each Cd concentration. Biochar application resulted in significant changes in extractable Cd concentrations only at the highest contamination level in both soils (Table 4). As a result, a 3% application of biochar reduced the extractable Cd content by 20.1% in SL and 8.4% in CL soils.
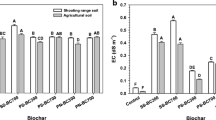
Biochar applications in SL and CL soils caused a slight yet significant increase in soil pH (Table 4), as water interaction with biochar releases hydroxide groups and alkaline carbonates (Fidel et al., 2017). Compared to previous research reporting a decrease in soil EC after lower-EC DPS biochar-treatment (Paz-Ferreiro et al., 2017), this study found no significant change in soil EC post biochar application.
An increased DHG activity was achieved in SL soils, alongside the significant decreases in Cd availability, indicative of elevated microbial activity with increasing biochar doses in SL40 and SL80 soils (Fig. 2). In SL20 soil, only the 3% biochar-treated soil significantly increased the DHG activity, the 1% biochar-treated soil being not different from the control. BGLU activities also rose proportionally, showing strong correlations with biochar dose, particularly in SL soils. In contrast, CL soils showed significantly increased enzymatic activity only at 20 mg Cd per kg. Although an upward trend in DHG and BGLU was observed in CL40 soil with increasing biochar doses, this did not reach statistical significance due to the high variability between samples.
Changes in the dehydrogenase (DHG) and betaglucosidase (BGLU) enzyme activities of sandy loam and clay loam soils for different biochar application and Cd contamination rates; 0, 1 and 3 correspond to % (w/w) of biochar application rate; lowercase letters indicate significant differences between BGLU values, while capital letters indicate the same for DHG values, based on each biochar application and related Cd contamination rate (p < .01); r values represent the correlations between DHG and BGLU with the biochar application rate for each specific contamination scenario. The p-values of correlations below .05 are precisely presented; whiskers represent mean ±1.5*standard deviation
3.2 Changes in Plant Stress Parameters
Biochar addition significantly increased chlorophyll a content in plants grown in CL20 soils and, more substantially, in CL80 and SL80 soils (Fig. 3a). This aligns with changes in dry matter content and RWC in SL80 and CL80 plants, significantly correlated with biochar doses (Fig. 3c, d). Dose-dependent differences were seen in chlorophyll a and dry matter content in SL80 soil and dry matter content in CL80 soil based on the linear correlation between biochar dose and the measured values. Chlorophyll b responded inconsistently to biochar applications (Fig. 3b). The RWC resulted in significant increases in water potential for plants grown in 3% biochar-treated soil, except for those grown in SL20 and CL40 soils (Fig. 3d).
Changes in chlorophyll a (a), chlorophyll b (b), dry matter content (c), and relative water content (d) for different biochar application and Cd contamination rates; 0, 1 and 3 correspond to % (w/w) of biochar application rate; lowercase letters indicate significant changes in the specific stress parameter based on each biochar application and related Cd contamination rate (p < .05); r values represent the correlations between the stress parameters and the biochar application rate for each specific contamination scenario; p-values of correlations below .05 are precisely presented
The MDA content of the plant leaves varied widely under biochar applications. Biochar incubation significantly reduced the MDA content of plants grown in soils SL40 and SL80, and the effect was dose-dependent (Fig. 4a); similar results were observed for plants grown in CL80 soil. However, IL rate was influenced only by 3% biochar application rate in SL40 and SL80 soils, while it was dependent to biochar dose in CL80 soil (Fig. 4b).
Contour graphs representing the changes in malondialdehyde (MDA) content (a) and the ion leakage (IL) rate (b) of wheat plants, based on each biochar application rate and the soil related Cd contamination rate; precise values for the related parameters are provided with the visual representation by contour plots; lowercase letters represent significant differences in the specific stress parameter based on each biochar application and Cd contamination rate (p < .05)
Increasing Cd contamination had a major influence on stress parameters as expected, especially in soils without biochar (Table 5). Based on these correlations, plants grown in SL soils might take advantage of greater stress mitigation. With the application of biochar (1% and 3% dose), the correlations between stress parameters and Cd concentrations were generally weaker and less significant, compared to the soils without biochar. The MDA values did not have any significant relationship with the Cd concentrations in SL soils receiving biochar; this fact can be supported by the findings presented in Fig. 4a, where the MDA content remained pretty similar across Cd concentrations of 20 to 80 mg kg-1, connected to the increase in the rate of biochar application. Additionally, in plants grown in SL soils, the dry matter content and chlorophyll a content did not exhibit the same negative correlation with increasing Cd contamination rates at 3% biochar dose as observed in plants grown in control soils and in soils with a 1% biochar application. On the other hand, in CL soils, the significance of correlations between stress parameters and Cd concentrations showed a similar decrease with increasing biochar doses which was, nevertheless, still significant at the 3% biochar dose.
Given the significant responsiveness of stress parameters IL rate and RWC, further investigations have been conducted. Our analysis indicated that these two variables contributed considerably to the variance as illustrated by the principal component analysis (Fig. S1). These stress parameters, in conjunction with the experimental design factors such as biochar dose, contamination rate, and soil type, were utilized in a general classification/regression trees (GCRT) model. This approach allowed us to predict other plant physiological parameters including chlorophyll a, MDA, dry matter content, as well as dry and fresh biomass. These initial predictions were further refined using three machine learning algorithms (MLAs): ANN, SVM and MARS. MLAs were trained on actual values of IL, RWC, and design parameters to enhance prediction quality.
The improved correspondence in terms of regression coefficient (R2) and RMSD values for the estimations was evident in MDA (R2 = 0.81, RMSD = 0.81, achieved via GCRT+SVM), dry matter content (R2 = 0.87, RMSD = 0.61, achieved via GCRT+SVM), chlorophyll a (R2 = 0.84, RMSD = 0.30, achieved via GCRT+ANN), and dry biomass (R2 = 0.89, RMSD = 0.007, achieved via GCRT+SVM). Estimations achieved by fitting these MLAs linearly are provided in Fig. S2. The Predictive Model Markup Language (PMML) codes for the most accurate SVM predictions are included in Table S4. This provision may facilitate reproducibility and retesting with various machine learning algorithms; the performance conditions for each model are outlined in Table S2. The observed and predicted values, along with their correspondence, as determined by each algorithm, in addition to the specifications of the initial GCRT predictions, are provided in the accompanying supplementary spreadsheets (refer to the file ‘Supplementary_spreadsheets.xlsx’).
4 Discussion
4.1 Soil Cd Availability, Physicochemical Properties and Enzymatic Activity
The soil types, typic xerofluvents (SL soil) and vertic xerofluvents (CL soil), predominantly found in alluvial environments, share several characteristics such as efficient drainage capabilities and high base saturation levels (Abd-Elmabod et al., 2017). However, vertic xerofluvents distinguish themselves with a notable content of expansive clay minerals, a feature that can significantly influence the dynamics of Cd adsorption. During the aging period, in both typic and vertic xerofluvents, Cd has the potential to bind to the surface of soil particles, subsequently decreasing its bioavailability. Considering the relatively neutral pH of untreated SL soil and the slightly alkaline pH of untreated CL soil, observed both before and after the 7-mo aging and incubation period, it's notable that both conditions can facilitate Cd immobilization due to increased binding sites in soil with diminished affinitive competition for these sites. Under these ease conditions for immobilization, the reduction in SL soil is primarily achieved through Cd's adsorption onto organic matter, iron/aluminium oxides, or silicate clays (Gong & Donahoe, 1997). On the other hand, CL soil can enhance Cd adsorption due to their high mineral associated CEC and specific surface area (Kovda, 2020; Table 3). The aging process after artificial contamination is essential when assessing biochar's remediation potential. This period lets metals, like Cd, equilibrate within the soil matrix, providing a more representative view of field contamination. As shown in our study, this process has a notable effect on metal bioavailability in different soil types. Therefore, including an aging step is beneficial for accurate insights into biochar's efficacy.
In biochar-treated soils, the decrease in Cd availability at the highest contamination rate is considered to be associated with the electronegativity of biochar. A high electronegativity of biochar is known to enhance the electrostatic attraction of positively charged ions, with the intensity augmenting in correlation with the initial concentrations of heavy metals (Dai et al., 2016). Previous studies have shown that DPS biochar is effective in remediating Ni- and Zn-contaminated soils, with immobilization efficiencies of 32.6% DTPA-extractable Ni; 89% water-soluble Ni and over 95% (water-soluble Zn) reported (Méndez et al., 2014; Paz-Ferreiro et al., 2017). Importantly, the chosen artificial contamination rates were 1000 mg kg-1 for Ni and 2000 mg kg-1 for Zn.
While previous studies haven't specifically assessed the dose-dependent impact of DPS biochar, similar trends have been observed with other biochars (Yang et al., 2016; Simms et al., 2020). The adsorption efficiency of biochar may be affected in clayey soils due to electrostatic interactions between heavy metals, negatively charged clay minerals, and biochar’s exchange complexes (Uchimiya et al., 2011), and possibly by the formation of clay-bound organic carbon complexes (Wattel-Koekkoek et al., 2001). The soil solution's cationic composition might also influence this effect (Gogoi et al., 2021). This inference aligns with the smaller pH increase seen in clayey soils after biochar application (Table 4), indicating differences in buffering and cation exchange capacities.
Biochar's effectiveness in immobilizing heavy metals is tied to its pore structure, surface area, and surface functional groups (Zhou et al., 2018). These factors influence the availability of heavy metal adsorption sites and the interactions between biochar and metals, helping to explain the varied immobilization efficiency between SL and CL soils. Clayey soils offer more adsorption sites for heavy metals. This leads to a relative decrease in the amount of Cd immobilized within the biochar matrix (El-Naggar et al., 2022). This consideration aligns with the specific surface area of the CL soil, which showed a comparatively smaller difference in this attribute with biochar than did the SL soil (Table 3). Upon quantitative calculation, the change in specific surface area of SL soils corresponds to an increase of 1.64% after a 3% biochar application, the increase was only 0.22% for CL soils.
The observed reductions in heavy metal availability are known to be associated with the adsorption reactions of metals with biochars oxygen-containing functional groups (Penido et al., 2019) and the subsequent formation of precipitates with carbonate and phosphate compounds (Faheem et al., 2020). Many biochars, due to their alkaline nature, elevate the pH of the soil solution and increase the hydroxyl density on their surface. This promotes the formation of hydroxyl-metal precipitates, thereby reducing heavy metal availability (Blenis et al., 2023). Cd, being a positively charged ion, adheres to negatively charged mineral surfaces up to the point where the zero charge (PZC) is surpassed. Considering vermiculite's higher PZC (8±1) compared to quartz's PZC (2.0) (Wen et al., 2013; Wang et al., 2018), clayey soils might be more efficient at sequestering Cd, influencing Cd immobilization within the biochar matrix. Additionally, the significant carbonate content in DPS biochar might support a higher soil pH, thus preventing the re-dissolution of immobilized Cd (Ippolito et al., 2017). The importance of this characteristic of DPS biochar becomes evident when considering recommendations to add lime to biochar treatments to meet long-term remediation goals (Phillips et al., 2016). As pH values increase and microbial activity is stimulated, the propensity for Cd to bind with inorganic carbon also rises, making the formation of CdCO3 (otavite) more likely (Yan et al., 2023).
An intriguing possibility is that the high CaCO3 content in the CL soil (27.3% compared to 8.2% in SL soil, Table 3) might have influenced the ability of biochar to bind to Cd. CaCO3 can also bind to heavy metals (Bashir et al., 2019). The high CaCO3 content in CL soil might influence biochar's ability to bind to Cd, potentially competing with biochar's binding sites. However, the differences between SL and CL soils cannot be solely attributed to the variation in CaCO3 content, as previously discussed. This is due to the high number of exchange complexes and the extensive specific surface area, which result from the presence of the smectite group of clay minerals in CL soil.
Several studies echo the application of biochars to reduce heavy metal availability in soils. Bashir et al. (2018) used 1.5% (w/w) sugarcane bagasse biochar to treat Cd and Cr in coarse-textured soil, decreasing DTPA-extracted Cd and Cr contents by 62.5% and 85%, respectively, over a 4-mo incubation period. Similarly, Moradi & Karimi (2021) employed 2% (w/w) common reed biochar on sandy loam soils contaminated with Cd, leading to a 22.1% to 28.6% reduction in DTPA-extractable Cd over 3-mo. Ogunkunle et al. (2021) reported a decrease in readily extractable Cd fractions using cocoa pod biochar (1% and 3% [w/w]) on contaminated soils over six weeks, yielding reductions of 24.8% and 47.1% respectively. These studies suggest a link between biochar application and elevated pH levels, similar to our findings. In summary, biochar’s efficacy in remediating Cd-contaminated soils depends on multiple factors like the type and origin of the biochar, the length of the incubation period, the presence of an aging period after the artificial contamination and the soil's physical and chemical properties.
Soil organic matter content and composition are known to impact toxic metal immobilization (Uchimiya et al., 2010; Kumpiene et al., 2019). In the current study, the organic matter content in both SL and CL soils was similar (~2%), due to slow accumulation under the East Mediterranean region’s semi-arid conditions. This finding coincides with the report of Hu et al. (2020), which indicated that Cd immobilization through biochar application is the most effective when 2% to 3% soil organic matter is present. While soil organic matter can form complexes with heavy metals, affecting their mobility and bioavailability (O’Connor et al., 2018), this study’s design attempts to emphasize the influence of textural differences on remediation efficacy over the impact of organic matter content. Biochar’s addition to soil, increasing organic matter and soluble nutrient content, can stimulate DHG activity (Ouyang et al. 2014) which plays a role in the biological oxidation of organic matter by hydrogen transfer from the organic substrate to inorganic acceptors (Zhang et al., 2010). The increase in DHG activity, combined with a significant reduction in extractable Cd content in SL80 soil, explains the strong correlation between biochar dose and enzyme activities. Soil characteristics like water-holding capacity, nutrient retention, and pore structure affect microbial-soil interactions; These collective factors can also cause the observed disparities between SL and CL soils, since the finer pore structure of CL soil may restrict microbial growth (Bucka et al., 2021). Specifically, the observed differences in response between soil types at higher contamination rates may stem from the microorganisms in SL soil utilizing nutrients and water more easily, particularly due to higher free soluble-carbon and possibly more reduced water-soluble Cd in the soil solution compared to that in CL soil after the biochar treatment.
DHG is a cellular enzyme (Saha et al., 2012); whereas BGLU, as an extracellular enzyme, catalyses biogeochemical reactions in the soil and contributes to the microbial degradation of cellulose to glucose (Valaskova & Baldrian, 2006). Soils, when altered in terms of bio-adsorption properties by organic amendments, can exhibit lower enzyme activities of BGLU or phosphatase. This is attributed to an increase in the soil surface area that facilitates direct enzyme adsorption (Foster et al., 2018), especially evident in the less responsive CL40 and CL80 soils. Overall, it can be suggested that the findings of the current study agree with several similar studies (Lu et al., 2015; Bashir et al., 2018; Moradi & Karimi, 2021; Ogunkunle et al., 2021). Given the observed decrease in Cd bioavailability in both SL80 and CL80 soils and the heightened microbial activity in SL80 soil as indicated by enzymatic activities, several mechanisms might influence the speciation or bioavailability of Cd. These mechanisms include biosorption, bioaccumulation, biotransformation through redox reactions, and the formation of organo-mineral complexes (Gul et al., 2021).
4.2 Plant Physiology
The results of the current study on stress parameters and soil extractable Cd reveal a proportional relationship between Cd immobilization and plant responses, thereby providing a comprehensive understanding of the experimental design and achieved outcomes.
Cd disrupts photosynthetic pigment synthesis, like chlorophyll and carotenoid, reducing cellular productivity (Baszyńki et al., 1980; Prasad, 1995). The results indicated that the treatment enhances photosynthetic capacity under Cd stress, primarily at high concentrations, corroborated by the dose-dependent dry matter increase in 80 mg Cd kg-1 treated plants due to increased cellular productivity. Chlorophyll b, which responded inconsistently to biochar applications, is a secondary pigment for capturing and transferring light energy (Eggink et al., 2001; Tyutereva et al., 2014). This pigment can exhibit different level of sensitivity to abiotic stress factors as observed in the current study (Yang et al., 2020). There is currently no previous scientific study on the influence of DPS biochar in an analogous concept, however, it may be noted that Bashir et al. (2018) reported that sugarcane bagasse biochar promoted greater production of dry matter in plant shoot tissue under Cd stress, with an increase of 35% in dry yield. Similarly, Ogunkunle et al. (2021) investigated changes in bioactive compounds such as chlorophyll a and b in tomato leaves after incubating Cd-contaminated soil with pod-derived biochar and found significant increases in chlorophyll a and b contents in plants supported by biochar application under Cd stress.
The RWC is one of the common parameters that provides a brief idea about the water status of the plants under stress conditions. Results of the present study indicated that Cd-induced oxidative stress on the plants is reduced due to the remediation success of biochar. Heavy metal originated stress including Cd, decreases RWC in plants (Hasanuzzaman et al., 2018; Ghasemzadeh et al., 2022). The ameliorative effect of different biochars on the heavy-metal stress-induced plant water status has been shown in different plant species (Zulqurnain Haider et al., 2019; Abd El-Mageed et al., 2020; Bashir et al., 2021; Khosropour et al., 2022). Cd, being a non-redox metal, does not participate in the electron transfer chain independently. Instead, it induces oxidative stress by disrupting the plant's antioxidant defense system (Chaoui et al., 1997; Benavides et al., 2005). Cd stress can overpower antioxidant defense mechanisms in plants, such as superoxide dismutase, catalase, and peroxidase, triggering oxidative stress. This stress, in turn, leads to lipid peroxidation and MDA production (Cuypers et al., 2023). Typically, damage to plant cell membranes triggers an upsurge in the IL rate. This event, coupled with the corresponding decrease in chlorophyll a content, precipitates a reduction in the cellular glucose content. Given that glucose can function as an osmolyte, its fluctuation - either increase or decrease - manifests in corresponding changes in the RWC values.
Increased rates of MDA and IL are well-known indicators of oxidative stress, under the influence of heavy metal pollution (Farooq et al., 2020; Chen et al., 2022), as it was observed in the current study with proportional increases to contamination rate in nontreated soils. However, biochar addition disturbed the linearity of stress responses, particularly in SL soil. The biochar dose had a strong negative correlation with the IL rate, with an r value of -.810 (p < .001) in the CL80 soil and a moderately negative correlation with the same parameter in the SL80 soil (r = -.676, p = .006). MDA values had a similar correlative relationship with increasing biochar dose in SL80 and CL80 soils, with r values of -.575 (p = .009) and -.648 (p = .025), respectively. Zhu et al. (2020) also reported that biochar was able to mitigate Cd stress in cotton (Gossypium hirsutum L.), as a mutual decrease in the leaf IL rate and the MDA content after the application of 3% biochar in artificially polluted soils (1, 2, 4 mg Cd kg-1). Younis et al. (2016), focusing on a higher range of contamination, found that biochar reduced MDA values in spinach leaves grown in 25, 50, and 100 mg kg-1 Cd-spiked soils (3% and 5% biochar applied). The greatest reduction in MDA was observed in plants grown in soils contaminated with 25 mg kg-1 with 5% biochar application, followed by plants in 100 mg kg-1 Cd contamination. The effect was not significant in plants grown in moderately contaminated 50 mg kg-1 Cd-spiked soils. This pattern was similar to the results observed here for moderately contaminated CL40 plants, which were not affected by biochar applications in terms of leaf MDA content; however, the plants grown in CL20 and CL80 soils showed a clear decrease in MDA content with the application of 3% biochar.
The parameters of IL and RWC, given their variance explaining capacity in evaluating stress conditions in highly contaminated regions (Fig. S1), are particularly promising for future environmental modeling efforts. Precise predictions of biomass production and stress metrics (refer to Fig. S2 and supplementary spreadsheets) can not only augment our grasp of plant-soil-contaminant interplays but can also inform effective strategies for environmental rehabilitation in polluted mining locales. Such future research with MLAs may encompass diverse variables like plant species, types and doses of biochar, contamination levels, and soil types.
5 Conclusions
This study explored the use of DPS biochar to remediate Cd contamination in soils that were artificially polluted and aged to simulate Cd availability in severely contaminated mining and industrial areas. The application of DPS biochar was considerably successful in reducing Cd extractability and toxicity, attributable to its role in elevating soil pH, promoting microbial activity, and increasing specific surface area. This remediation effect was particularly evident in SL soil, where enhancements in pH and microbial activity were more pronounced compared to CL soil. Furthermore, the study underscores the dependency of biochar's remedial influence on the contamination level, with the most significant impact observed in soils contaminated with 80 mg kg-1 of Cd.
The use of DPS biochar also demonstrated potential in mitigating plant stress in similar environments, as evidenced by increased chlorophyll content, RWC, and dry matter content, alongside a reduction in oxidative stress. However, the study's findings are limited by the 1-mo incubation period and the variability of biochar and experimental soil properties, particularly related to the initial soil pH (neutral to slightly alkaline), texture (clay loam and sandy loam soils) and organic matter content (relatively low). On the other hand, in assessing biochar's remediation potential, our study emphasized the importance of the aging process after artificial contamination, as it significantly affects metal bioavailability and offers a more accurate representation of field contamination.
The relatively simple production conditions of DPS biochar, which require low technology, present a strategy both for coping with solid-waste buildup and for addressing the gradual increase in Cd accumulation in highly contaminated soils. Biochar derived from DPS shows potential as a sustainable and eco-friendly remediation material for reducing Cd bioavailability in particularly coarser and heavily contaminated SL soil, indicating a positive impact on possible co-phytoremediation efforts as well. Further research is needed to investigate long-term effects and determine optimal application rates and incubation periods for different doses of biochar and contamination rates. In addition, the possible changes in soil nutrient availability and microbial diversity, along with the translocation and bioconcentration factors of plants under the same environmental remediation concept, become prominent topics for the optimization of DPS biochar use.
Data Availability
Plant data can be found in the file ‘Supplementary_spreadsheets.xlsx’. All additional data supporting the findings will be made available upon reasonable request.
References
Abd El-Mageed, T. A., Abdurrahman, H. A., & Abd El-Mageed, S. A. (2020). Residual acidified biochar modulates growth, physiological responses, and water relations of maize (Zea mays) under heavy metal–contaminated irrigation water. Environmental Science and Pollution Research, 27, 22956–22966. https://doi.org/10.1007/s11356-020-08847-5
Abd-Elmabod, S. K., Jordán, A., Fleskens, L., Phillips, J. D., Muñoz-Rojas, M., van der Ploeg, M., ... & de la Rosa, D. (2017). Modeling agricultural suitability along soil transects under current conditions and improved scenario of soil factors. In Soil mapping and process modeling for sustainable land use management (pp. 193-219). Elsevier. https://doi.org/10.1016/B978-0-12-805200-6.00007-4
Abida, K., Boudabbous, K., Ayed, L. B., Barbouchi, M., Bachwell, S., & Benzina, N. K. (2023). Industrial deinking paper sludge waste: toxicity risk and health effects assessment of heavy metals by USEtox model. GSC Advanced Research and Reviews, 15(1), 115–123. https://doi.org/10.30574/gscarr.2023.15.1.0125
Adetunji, A. T., Lewu, F. B., Mulidzi, R., & Ncube, B. (2017). The biological activities of β-glucosidase, phosphatase and urease as soil quality indicators: a review. Journal of Soil Science and Plant Nutrition, 17(3), 794–807. https://doi.org/10.4067/S0718-95162017000300018
Arnon, D. I. (1949). Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiology, 24(1), 1–15. https://doi.org/10.1104/pp.24.1.1
Arslan, Ş, & Çelik, M. (2015). Assessment of the pollutants in soils and surface waters around Gümüşköy silver mine (Kütahya, Turkey). Bulletin of Environmental Contamination and Toxicology, 95, 499–506. https://doi.org/10.1007/s00128-015-1613-6
ASTM International. (2007). ASTM D1762-84: Standard test method for chemical analysis of wood charcoal. In Subcommittee D07.01 (Eds.), Book of standards (vol. 04.10., pp. 1–2). https://doi.org/10.1520/D1762-84R07
Bajpai, P. (2015). Generation of waste in pulp and paper mills. In Management of pulp and paper mill waste, (pp. 9-17). Springer, Cham. https://doi.org/10.1007/978-3-319-11788-1_2
Baruah, N., Mondal, S. C., Farooq, M., & Gogoi, N. (2019). Influence of heavy metals on seed germination and seedling growth of wheat, pea, and tomato. Water, Air, & Soil Pollution, 230, 1–15. https://doi.org/10.1007/s11270-019-4329-0
Baryla, A., Carrier, P., Franck, F., Coulomb, C., Sahut, C., & Havaux, M. (2001). Leaf chlorosis in oilseed rape plants (Brassica napus) grown on cadmium-polluted soil: causes and consequences for photosynthesis and growth. Planta, 212, 696–709. https://doi.org/10.1007/s004250000439
Bashir, S., Hussain, Q., Akmal, M., Riaz, M., Hu, H., Ijaz, S. S., ... & Ahmad, M. (2018). Sugarcane bagasse-derived biochar reduces the cadmium and chromium bioavailability to mash bean and enhances the microbial activity in contaminated soil. Journal of Soils and Sediments, 18, 874-886. https://doi.org/10.1007/s11368-017-1796-z
Bashir, M. A., Wang, X., Naveed, M., Mustafa, A., Ashraf, S., Samreen, T., ... & Jamil, M. (2021). Biochar mediated-alleviation of chromium stress and growth improvement of different maize cultivars in tannery polluted soils. International Journal of Environmental Research and Public Health, 18(9), 4461. https://doi.org/10.3390/ijerph18094461
Bashir, M. A., Rehim, A., Liu, J., Imran, M., Liu, H., Suleman, M., & Naveed, S. (2019). Soil survey techniques determine nutrient status in soil profile and metal retention by calcium carbonate. Catena, 173, 141–149. https://doi.org/10.1016/j.catena.2018.10.015
Baszyńki, T., Wajda, L., Krol, M., Wolińska, D., Krupa, Z., & Tukendorf, A. (1980). Photosynthetic activities of cadmium-treated tomato plants. Physiologia Plantarum, 48(3), 365–370. https://doi.org/10.1111/j.1399-3054.1980.tb03269.x
Battaglia, A., Calace, N., Nardi, E., Petronio, B. M., & Pietroletti, M. (2007). Reduction of Pb and Zn bioavailable forms in metal polluted soils due to paper mill sludge addition: Effects on Pb and Zn transferability to barley. Bioresource Technology, 98(16), 2993–2999. https://doi.org/10.1016/j.biortech.2006.10.007
Benavides, M. P., Gallego, S. M., & Tomaro, M. L. (2005). Cadmium toxicity in plants. Brazilian Journal of Plant Physiology, 17, 21–34. https://doi.org/10.1590/S1677-04202005000100003
Blenis, N., Hue, N., Maaz, T. M., & Kantar, M. (2023). Biochar production, modification, and its uses in soil remediation: a review. Sustainability, 15(4), 3442. https://doi.org/10.3390/su15043442
Bolan, N., Kunhikrishnan, A., Thangarajan, R., Kumpiene, J., Park, J., Makino, T., ... & Scheckel, K. (2014). Remediation of heavy metal (loid) s contaminated soils–to mobilize or to immobilize?. Journal of Hazardous Materials, 266, 141-166. https://doi.org/10.1016/j.jhazmat.2013.12.018
Bonoli, A., & Dall’Ara, A. (2012). A bioremediation case of an ex-quarry area restored by paper sludge. Journal of Biotechnology, 157(4), 499–504. https://doi.org/10.1016/j.jbiotec.2011.08.010
Boussen, S., Soubrand, M., Bril, H., Ouerfelli, K., & Abdeljaouad, S. (2013). Transfer of lead, zinc and cadmium from mine tailings to wheat (Triticum aestivum) in carbonated Mediterranean (Northern Tunisia) soils. Geoderma, 192, 227–236. https://doi.org/10.1016/j.geoderma.2012.08.029
Bouyoucos, G. J. (1962). Hydrometer method improved for making particle size analyses of soils 1. Agronomy Journal, 54(5), 464–465.
Bremner, J. M. (1965). Total nitrogen. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties (pp. 1149-1178). American Society of Agronomy, Inc., Soil Science Society of America, Inc. https://doi.org/10.2134/agronmonogr9.2.2ed.c31
Bucka, F. B., Felde, V. J., Peth, S., & Kögel-Knabner, I. (2021). Disentangling the effects of OM quality and soil texture on microbially mediated structure formation in artificial model soils. Geoderma, 403, 115213. https://doi.org/10.1016/j.geoderma.2021.115213
Calace, N., Campisi, T., Iacondini, A., Leoni, M., Petronio, B. M., & Pietroletti, M. (2005). Metal-contaminated soil remediation by means of paper mill sludges addition: chemical and ecotoxicological evaluation. Environmental Pollution, 136(3), 485–492. https://doi.org/10.1016/j.envpol.2004.12.014
Camberato, J. J., Gagnon, B., Angers, D. A., Chantigny, M. H., & Pan, W. L. (2006). Pulp and paper mill by-products as soil amendments and plant nutrient sources. Canadian Journal of Soil Science, 86(4), 641–653. https://doi.org/10.4141/S05-120
Chakraborty, D., Dahiya, S., Amulya, K., Srivastav, V., & Mohan, S.V. (2019) Valorization of paper and pulp waste: opportunities and prospects of biorefinery. In Industrial and Municipal Sludge (pp. 623-656). Butterworth-Heinemann. https://doi.org/10.1016/B978-0-12-815907-1.00027-1
Chaoui, A., Mazhoudi, S., Ghorbal, M. H., & El Ferjani, E. (1997). Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L.). Plant Science, 127(2), 139–147. https://doi.org/10.1016/S0168-9452(97)00115-5
Chapman, H. D. (1965). Cation‐exchange capacity. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties (pp. 891-901). American Society of Agronomy, Inc., Soil Science Society of America, Inc.. https://doi.org/10.2134/agronmonogr9.2.c6
Chen, F., Bashir, A., ur Rehman, M. Z., Adrees, M., Qayyum, M. F., Ma, J., ... & Ali, S. (2022). Combined effects of green manure and zinc oxide nanoparticles on cadmium uptake by wheat (Triticum aestivum L.). Chemosphere, 298, 134348. https://doi.org/10.1016/j.chemosphere.2022.134348
Chin-Pampillo, J. S., Alfaro-Vargas, A., Rojas, R., Giacomelli, C. E., Perez-Villanueva, M., Chinchilla-Soto, C., ... & Domene, X. (2021). Widespread tropical agrowastes as novel feedstocks for biochar production: characterization and priority environmental uses. Biomass Conversion and Biorefinery, 11, 1775-1785. https://doi.org/10.1007/s13399-020-00714-0
Cuypers, A., Vanbuel, I., Iven, V., Kunnen, K., Vandionant, S., Huybrechts, M., & Hendrix, S. (2023). Cadmium-induced oxidative stress responses and acclimation in plants require fine-tuning of redox biology at subcellular level. Free Radical Biology and Medicine, 199, 81–96. https://doi.org/10.1016/j.freeradbiomed.2023.02.010
Dai, Z., Meng, J., Shi, Q., Xu, B., Lian, Z., Brookes, P. C., & Xu, J. M. (2016). Effects of manure-and lignocellulose-derived biochars on adsorption and desorption of zinc by acidic types of soil with different properties. European Journal of Soil Science, 67(1), 40–50. https://doi.org/10.1111/ejss.12290
Eggink, L. L., Park, H., & Hoober, J. K. (2001). The role of chlorophyll b in photosynthesis: hypothesis. BMC Plant Biology, 1, 1–7. https://doi.org/10.1186/1471-2229-1-2
El-Naggar, A., Chen, Z., Jiang, W., Cai, Y., & Chang, S. X. (2022). Biochar effectively remediates Cd contamination in acidic or coarse-and medium-textured soils: A global meta-analysis. Chemical Engineering Journal, 442, 136225. https://doi.org/10.1016/j.cej.2022.136225
Enwezor, W. O. (1976). The mineralization of nitrogen and phosphorus in organic materials of varying C: N and C: P ratios. Plant and Soil, 44, 237–240. https://doi.org/10.1007/BF00016972
Faheem, Du, J., Bao, J., Hassan, M. A., Irshad, S., Talib, M. A., & Zheng, H. (2020). Efficient capture of phosphate and cadmium using biochar with multifunctional amino and carboxylic moieties: kinetics and mechanism. Water, Air, & Soil Pollution, 231, 1-16. https://doi.org/10.1007/s11270-019-4389-1
Fan, S., & Blake, T. J. (1994). Abscisic acid induced electrolyte leakage in woody species with contrasting ecological requirements. Physiologia Plantarum, 90(2), 414–419. https://doi.org/10.1111/j.1399-3054.1994.tb00407.x
Farooq, M., Ullah, A., Usman, M., & Siddique, K. H. (2020). Application of zinc and biochar help to mitigate cadmium stress in bread wheat raised from seeds with high intrinsic zinc. Chemosphere, 260, 127652. https://doi.org/10.1016/j.chemosphere.2020.127652
Fidel, R. B., Laird, D. A., Thompson, M. L., & Lawrinenko, M. (2017). Characterization and quantification of biochar alkalinity. Chemosphere, 167, 367–373. https://doi.org/10.1016/j.chemosphere.2016.09.151
Foster, E. J., Fogle, E. J., & Cotrufo, M. F. (2018). Sorption to biochar impacts β-glucosidase and phosphatase enzyme activities. Agriculture, 8(10), 158. https://doi.org/10.3390/agriculture8100158
Ghasemzadeh, N., Iranbakhsh, A., Oraghi-Ardebili, Z., Saadatmand, S., & Jahanbakhsh-Godehkahriz, S. (2022). Cold plasma can alleviate cadmium stress by optimizing growth and yield of wheat (Triticum aestivum L) through changes in physio-biochemical properties and fatty acid profile. Environmental Science and Pollution Research, 29(24), 35897–35907. https://doi.org/10.1007/s11356-022-18630-3
Gogoi, L., Narzari, R., Chutia, R. S., Borkotoki, B., Gogoi, N., & Kataki, R. (2021). Remediation of heavy metal contaminated soil: Role of biochar. In Advances in Chemical Pollution, Environmental Management and Protection (Vol. 7, pp. 39-63). Elsevier. https://doi.org/10.1016/bs.apmp.2021.08.002
Gong, C., & Donahoe, R. J. (1997). An experimental study of heavy metal attenuation and mobility in sandy loam soils. Applied Geochemistry, 12(3), 243–254. https://doi.org/10.1016/S0883-2927(96)00049-2
Gul, I., Manzoor, M., Hashim, N., Shah, G. M., Waani, S. P. T., Shahid, M., ... & Arshad, M. (2021). Challenges in microbially and chelate-assisted phytoextraction of cadmium and lead–A review. Environmental Pollution, 287, 117667. https://doi.org/10.1016/j.envpol.2021.117667
Hasanuzzaman, M., Nahar, K., Rahman, A., Mahmud, J. A., Alharby, H. F., & Fujita, M. (2018). Exogenous glutathione attenuates lead-induced oxidative stress in wheat by improving antioxidant defense and physiological mechanisms. Journal of Plant Interactions, 13(1), 203–212. https://doi.org/10.1080/17429145.2018.1458913
Hoffmann, G. G., & Dedeken, M. (1965). A method for the colorimetric determination of β-glucosidase activity in soil. Zeitschrift für Pflanzenernährung und Bodenkunde, 108(3), 193–198. https://doi.org/10.1002/jpln.19651080302
Hu, Y., Zhang, P., Yang, M., Liu, Y., Zhang, X., Feng, S., ... & Dang, X. (2020). Biochar is an effective amendment to remediate Cd-contaminated soils—a meta-analysis. Journal of Soils and Sediments, 20, 3884-3895. https://doi.org/10.1007/s11368-020-02726-9
Hunt, R., Causton, D. R., Shipley, B., & Askew, A. P. (2002). A modern tool for classical plant growth analysis. Annals of Botany, 90(4), 485–488. https://doi.org/10.1093/aob/mcf214
Ippolito, J. A., Berry, C. M., Strawn, D. G., Novak, J. M., Levine, J., & Harley, A. (2017). Biochars reduce mine land soil bioavailable metals. Journal of Environmental Quality, 46(2), 411–419. https://doi.org/10.2134/jeq2016.10.0388
Jalalabadi, T., Glenn, M., Tremain, P., Moghtaderi, B., Donne, S., & Allen, J. (2019). Modification of biochar formation during slow pyrolysis in the presence of alkali metal carbonate additives. Energy & Fuels, 33(11), 11235–11245. https://doi.org/10.1021/acs.energyfuels.9b02865
Kabir, E., Ray, S., Kim, K. H., Yoon, H. O., Jeon, E. C., Kim, Y. S., ... & Brown, R. J. (2012). Current status of trace metal pollution in soils affected by industrial activities. The Scientific World Journal, 2012, 916705. https://doi.org/10.1100/2012/916705
Kessler, A., Hedberg, J., Blomberg, E., & Odnevall, I. (2022). Reactive oxygen species formed by metal and metal oxide nanoparticles in physiological media—a review of reactions of importance to nanotoxicity and proposal for categorization. Nanomaterials, 12(11), 1922. https://doi.org/10.3390/nano12111922
Khosropour, E., Weisany, W., Tahir, N. A. R., & Hakimi, L. (2022). Vermicompost and biochar can alleviate cadmium stress through minimizing its uptake and optimizing biochemical properties in Berberis integerrima bunge. Environmental Science and Pollution Research, 29(12), 17476–17486. https://doi.org/10.1007/s11356-021-17073-6
Knudsen, D., Peterson, G.A., & Pratt, P. F. (1982). Lithium, sodium and potassium. In A.L. Page, R.H. Miller, & D.R. Keeney, (Eds.) Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties. (2nd ed., pp. 225–246). American Society of Agronomy, Inc., Soil Science Society of America, Inc.
Kováčik, J., Dresler, S., Babula, P., Hladký, J., & Sowa, I. (2020). Calcium has protective impact on cadmium-induced toxicity in lichens. Plant Physiology and Biochemistry, 156, 591–599. https://doi.org/10.1016/j.plaphy.2020.10.007
Kováčik, J., Dresler, S., Sowa, I., Babula, P., & Antunes, E. (2022). Calcium-enriched biochar modulates cadmium uptake depending on external cadmium dose. Environmental Pollution, 313, 120178. https://doi.org/10.1016/j.envpol.2022.120178
Kovda, I. (2020). Vertisols: Extreme features and extreme environment. Geoderma Regional, 22, e00312. https://doi.org/10.1016/j.geodrs.2020.e00312
Kumpiene, J., Antelo, J., Brännvall, E., Carabante, I., Ek, K., Komárek, M., ... & Wårell, L. (2019). In situ chemical stabilization of trace element-contaminated soil–Field demonstrations and barriers to transition from laboratory to the field–A review. Applied Geochemistry, 100, 335-351. https://doi.org/10.1016/j.apgeochem.2018.12.003
Lindsay, W. L., & Norvell, W. (1978). Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Science Society of America Journal, 42(3), 421–428. https://doi.org/10.2136/sssaj1978.03615995004200030009x
Liu, T., Chen, Z., Li, Z., Chen, G., Zhou, J., Chen, Y., ... & Chen, Z. (2021). Rapid separation and efficient removal of Cd based on enhancing surface precipitation by carbonate-modified biochar. ACS Omega, 6(28), 18253-18259. https://doi.org/10.1021/2Facsomega.1c02126
Lou, R., Wu, S., Lv, G., & Yang, Q. (2012). Energy and resource utilization of deinking sludge pyrolysis. Applied Energy, 90(1), 46–50. https://doi.org/10.1016/j.apenergy.2010.12.025
Lu, Q., Xu, Z., Xu, X., Liu, L., Liang, L., Chen, Z., ... & Qiu, G. (2019). Cadmium contamination in a soil-rice system and the associated health risk: An addressing concern caused by barium mining. Ecotoxicology and Environmental Safety, 183, 109590. https://doi.org/10.1016/j.ecoenv.2019.109590
Méndez, A., Barriga, S., Fidalgo, J. M., & Gascó, G. (2009). Adsorbent materials from paper industry waste materials and their use in Cu (II) removal from water. Journal of Hazardous Materials, 165(1–3), 736–743. https://doi.org/10.1016/j.jhazmat.2008.10.055
Méndez, A., Paz-Ferreiro, J., Araujo, F., & Gasco, G. (2014). Biochar from pyrolysis of deinking paper sludge and its use in the treatment of a nickel polluted soil. Journal of Analytical and Applied Pyrolysis, 107, 46–52. https://doi.org/10.1016/j.jaap.2014.02.001
Moradi, N., & Karimi, A. (2021). Fe-Modified common reed biochar reduced cadmium (Cd) mobility and enhanced microbial activity in a contaminated calcareous soil. Journal of Soil Science and Plant Nutrition, 21, 329–340. https://doi.org/10.1007/s42729-020-00363-2
Moussa, H. R., & El-Gamal, S. M. (2010). Effect of salicylic acid pretreatment on cadmium toxicity in wheat. Biologia Plantarum, 54, 315–320. https://doi.org/10.1007/s10535-010-0054-7
Mulenga, C., Clarke, C., & Meincken, M. (2020). Physiological and growth responses to pollutant-induced biochemical changes in plants: A review. Pollution, 6(4), 827–848. https://doi.org/10.22059/poll.2020.303151.821
Nelson, R. E. (1983). Carbonate and gypsum. In Methods of soil analysis: Part 2 Chemical and microbiological properties (9, pp. 181-197). American Society of Agronomy, Inc., Soil Science Society of America, Inc.. https://doi.org/10.2134/agronmonogr9.2.2ed.c11
Nelson, D. W., & Sommers, L. E. (1996). Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 3 Chemical Methods (5th ed., pp. 961-1010). American Society of Agronomy, Inc., Soil Science Society of America, Inc.. https://doi.org/10.2136/sssabookser5.3.c34
O'Connor, D., Peng, T., Zhang, J., Tsang, D. C., Alessi, D. S., Shen, Z., ... & Hou, D. (2018). Biochar application for the remediation of heavy metal polluted land: a review of in situ field trials. Science of the Total Environment, 619, 815-826. https://doi.org/10.1016/j.scitotenv.2017.11.132
Ogunkunle, C. O., Falade, F. O., Oyedeji, B. J., Akande, F. O., Vishwakarma, V., Alagarsamy, K., ... & Fatoba, P. O. (2021). Short‐term aging of pod‐derived biochar reduces soil cadmium mobility and ameliorates cadmium toxicity to soil enzymes and tomato. Environmental Toxicology and Chemistry, 40(12), 3306-3316. https://doi.org/10.1002/etc.4958
Olsen, S. R., & Sommers, L. E. (1982). Phosphorus. In A. L. Page, R. H. Miller, & D. R. Keeney (Eds.), Methods of soil analysis. Part 2, Microbiological and biochemical properties (pp. 403-430). Soil Science Society of America, Madison, Wisconsin. https://doi.org/10.2134/agronmonogr9.2.2ed.c24
Ouyang, L., Tang, Q., Yu, L., & Zhang, R. (2014). Effects of amendment of different biochars on soil enzyme activities related to carbon mineralisation. Soil Research, 52(7), 706–716. https://doi.org/10.1071/SR14075
Palansooriya, K. N., Li, J., Dissanayake, P. D., Suvarna, M., Li, L., Yuan, X., ... & Ok, Y. S. (2022). Prediction of soil heavy metal immobilization by biochar using machine learning. Environmental Science & Technology, 56(7), 4187-4198. https://doi.org/10.1021/acs.est.1c08302
Palutoglu, M., Akgul, B., Suyarko, V., Yakovenko, M., Kryuchenko, N., & Sasmaz, A. (2018). Phytoremediation of cadmium by native plants grown on mining soil. Bulletin of Environmental Contamination and Toxicology, 100, 293–297. https://doi.org/10.1007/s00128-017-2220-5
Park, H. J., Kim, S. U., Jung, K. Y., Lee, S., Choi, Y. D., Owens, V. N., & Hong, C. O. (2021). Cadmium phytoavailability from 1976 through 2016: Changes in soil amended with phosphate fertilizer and compost. Science of The Total Environment, 762, 143132. https://doi.org/10.1016/j.scitotenv.2020.143132
Paz-Ferreiro, J., Plasencia, P., Gascó, G., & Méndez, A. (2017). Biochar from pyrolysis of deinking paper sludge and its use in the remediation of Zn-polluted soils. Land Degradation & Development, 28(1), 355–360. https://doi.org/10.1002/ldr.2597
Lu, H., Li, Z., Fu, S., Méndez, A., Gasco, G., & Paz-Ferreiro, J. (2015). Combining phytoextraction and biochar addition improves soil biochemical properties in a soil contaminated with Cd. Chemosphere, 119, 209–216. https://doi.org/10.1016/j.chemosphere.2014.06.024
Penido, E. S., Martins, G. C., Mendes, T. B. M., Melo, L. C. A., do Rosário Guimarães, I., & Guilherme, L. R. G. (2019). Combining biochar and sewage sludge for immobilization of heavy metals in mining soils. Ecotoxicology and Environmental Safety, 172, 326-333. https://doi.org/10.1016/j.ecoenv.2019.01.110
Phillips, C. L., Trippe, K. M., Whittaker, G., Griffith, S. M., Johnson, M. G., & Banowetz, G. M. (2016). Gasified grass and wood biochars facilitate plant establishment in acid mine soils. Journal of Environmental Quality, 45(3), 1013–1020. https://doi.org/10.2134/jeq2015.09.0470
Prasad, M. N. V. (1995). Cadmium toxicity and tolerance in vascular plants. Environmental and Experimental Botany, 35(4), 525–545. https://doi.org/10.1016/0098-8472(95)00024-0
Qu, J., Yuan, Y., Zhang, X., Wang, L., Tao, Y., Jiang, Z., ... & Zhang, Y. (2022). Stabilization of lead and cadmium in soil by sulfur-iron functionalized biochar: Performance, mechanisms and microbial community evolution. Journal of Hazardous Materials, 425, 127876. https://doi.org/10.1016/j.jhazmat.2021.127876
Rao, K. M., & Sresty, T. V. S. (2000). Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Science, 157(1), 113–128. https://doi.org/10.1016/S0168-9452(00)00273-9
Robbins, C. W., & Meyer, W. S. (1990). Calculating pH from EC and SAR values in salinity models and SAR from soil and bore water pH and EC data. Soil Research, 28(6), 1001–1011. https://doi.org/10.1071/SR9901001
Saha, S., Dutta, D., Karmakar, R., & Ray, D. P. (2012). Structure–toxicity relationship of chloroacetanilide herbicides: relative impact on soil microorganisms. Environmental Toxicology and Pharmacology, 34(2), 307–314. https://doi.org/10.1016/j.etap.2012.04.014
Schonfeld, M. A., Johnson, R. C., Carver, B. F., & Mornhinweg, D. W. (1988). Water relations in winter wheat as drought resistance indicators. Crop Science, 28(3), 526–531. https://doi.org/10.2135/cropsci1988.0011183X002800030021x
Shen, Y., & Yuan, R. (2021). Pyrolysis of agroforestry bio-wastes with Calcium/Magnesium oxides or carbonates–Focusing on biochar as soil conditioner. Biomass and Bioenergy, 155, 106277. https://doi.org/10.1016/j.biombioe.2021.106277
Shi, T., Zhang, Y., Gong, Y., Ma, J., Wei, H., Wu, X., ... & Hou, H. (2019). Status of cadmium accumulation in agricultural soils across China (1975–2016): From temporal and spatial variations to risk assessment. Chemosphere, 230, 136-143. https://doi.org/10.1016/j.chemosphere.2019.04.208
Simms, T., Chen, H., & Mahato, G. (2020). Dose-dependent effect of biochar as soil amendment on reducing copper phytotoxicity and mobility. International Journal of Environmental Research, 14, 751–759. https://doi.org/10.1007/s41742-020-00293-y
Singh, B., Dolk, M. M., Shen, Q., & Camps-Arbestain, M. (2017). Biochar pH, electrical conductivity and liming potential. In, Biochar: A guide to analytical methods (pp. 23-39). CSIRO Publishing. https://doi.org/10.1071/9781486305100
Singh, N. B., & Singh, N. P. (2007). Formation of CaO from thermal decomposition of calcium carbonate in the presence of carboxylic acids. Journal of Thermal Analysis and Calorimetry, 89, 159–162. https://doi.org/10.1007/s10973-006-7565-7
Singh, S. K., Kulkarni, S., Kumar, V., & Vashistha, P. (2018). Sustainable utilization of deinking paper mill sludge for the manufacture of building bricks. Journal of Cleaner Production, 204, 321–333. https://doi.org/10.1016/j.jclepro.2018.09.028
Soil Survey Staff (1999). Soil taxonomy: A basic system of soil classification for making and interpreting soil surveys. 2nd edition. In Agriculture Handbook 436 (p 869). Natural Resources Conservation Service, USDA, Washington DC, USA. https://doi.org/10.1111/j.1475-2743.2001.tb00008.x
Sridharan, A., Rao, G. V. (1972). Surface area determination of clays. Geotechnical Engineering, 3, 127–132.
Sun, J., Wang, P., Guo, Y., Hu, B., & Wang, X. (2023). Effect of biochar derived from co-pyrolysis of sewage sludge and rice straw on cadmium immobilization in paddy soil. Environmental Science and Pollution Research, 30, 74808–74819. https://doi.org/10.1007/s11356-023-26826-4
Thalmann, A. (1968). On the methodology for determining dehydrogenase activity in soil using triphenyltetrazolium chloride. Landwirtschaftliche Forschung, 21, 249–258.
Tsadik, Y. K. G., Hailu, A. M., Asfaw, S. L., & Mekonnen, Y. S. (2020). The effect of brewery sludge biochar on immobilization of bio-available cadmium and growth of Brassica carinata. Heliyon, 6(12). https://doi.org/10.1016/j.heliyon.2020.e05573
Tyutereva, E. V., Ivanova, A. N., & Voitsekhovskaja, O. V. (2014). On the role of chlorophyll b in ontogenetic adaptations of plants. Biology Bulletin Reviews, 4, 507–514. https://doi.org/10.1134/S2079086414060073
Uchimiya, M., Klasson, K. T., Wartelle, L. H., & Lima, I. M. (2011). Influence of soil properties on heavy metal sequestration by biochar amendment: 1. Copper sorption isotherms and the release of cations. Chemosphere, 82(10), 1431–1437. https://doi.org/10.1016/j.chemosphere.2010.11.050
Uchimiya, M., Lima, I. M., Klasson, K. T., & Wartelle, L. H. (2010). Contaminant immobilization and nutrient release by biochar soil amendment: roles of natural organic matter. Chemosphere, 80(8), 935–940. https://doi.org/10.1016/j.chemosphere.2010.05.020
Valaskova, V., & Baldrian, P. (2006). Degradation of cellulose and hemicelluloses by the brown rot fungus Piptoporus betulinus–production of extracellular enzymes and characterization of the major cellulases. Microbiology, 152(12), 3613–3622. https://doi.org/10.1099/mic.0.29149-0
Vance, E. D., Brookes, P. C., & Jenkinson, D. S. (1987). An extraction method for measuring soil microbial biomass C. Soil Biology and Biochemistry, 19(6), 703–707. https://doi.org/10.1016/0038-0717(87)90052-6
Vannucchi, F., Scartazza, A., Scatena, M., Rosellini, I., Tassi, E., Cinelli, F., & Bretzel, F. (2021). De-inked paper sludge and mature compost as high-value components of soilless substrate to support tree growth. Journal of Cleaner Production, 290, 125176. https://doi.org/10.1016/j.jclepro.2020.125176
Vashistha, P., Kumar, V., Singh, S. K., Dutt, D., Tomar, G., & Yadav, P. (2019). Valorization of paper mill lime sludge via application in building construction materials: A review. Construction and Building Materials, 211, 371–382. https://doi.org/10.1016/j.conbuildmat.2019.03.085
Wahid, A., Ghani, A., & Javed, F. (2008). Effect of cadmium on photosynthesis, nutrition and growth of mung bean. Agronomy for Sustainable Development, 28, 273–280. https://doi.org/10.1051/agro:2008010
Wang, C., Jia, Y., Wang, Q., Yan, F., Wu, M., Li, X., ... & Qiu, Z. (2022). Responsive change of crop-specific soil bacterial community to cadmium in farmlands surrounding mine area of Southeast China. Environmental Research, 214, 113748. https://doi.org/10.1016/j.envres.2022.113748
Wang, W., Cong, J., Deng, J., Weng, X., Lin, Y., Huang, Y., & Peng, T. (2018). Developing effective separation of feldspar and quartz while recycling tailwater by HF pretreatment. Minerals, 8(4), 149. https://doi.org/10.3390/min8040149
Wang, Z., Miao, R., Ning, P., He, L., & Guan, Q. (2021). From wastes to functions: a paper mill sludge-based calcium-containing porous biochar adsorbent for phosphorus removal. Journal of Colloid and Interface Science, 593, 434–446. https://doi.org/10.1016/j.jcis.2021.02.118
Wattel-Koekkoek, E. J. W., Van Genuchten, P. P. L., Buurman, P., & Van Lagen, B. (2001). Amount and composition of clay-associated soil organic matter in a range of kaolinitic and smectitic soils. Geoderma, 99(1–2), 27–49. https://doi.org/10.1016/S0016-7061(00)00062-8
Wen, Z. D., Gao, D. W., Li, Z., & Ren, N. Q. (2013). Effects of humic acid on phthalate adsorption to vermiculite. Chemical Engineering Journal, 223, 298–303. https://doi.org/10.1016/j.cej.2013.03.012
Wong, Y. J., Arumugasamy, S. K., Chung, C. H., Selvarajoo, A., & Sethu, V. (2020). Comparative study of artificial neural network (ANN), adaptive neuro-fuzzy inference system (ANFIS) and multiple linear regression (MLR) for modeling of Cu (II) adsorption from aqueous solution using biochar derived from rambutan (Nephelium lappaceum) peel. Environmental Monitoring and Assessment, 192, 1–20. https://doi.org/10.1007/s10661-020-08268-4
Xia, X., Wu, S., Zhou, Z., & Wang, G. (2021). Microbial Cd (II) and Cr (VI) resistance mechanisms and application in bioremediation. Journal of Hazardous Materials, 401, 123685. https://doi.org/10.1016/j.jhazmat.2020.123685
Xie, K., Xie, N., Liao, Z., Luo, X., Peng, W., & Yuan, Y. (2023). Bioaccessibility of arsenic, lead, and cadmium in contaminated mining/smelting soils: Assessment, modeling, and application for soil environment criteria derivation. Journal of Hazardous Materials, 443, 130321. https://doi.org/10.1016/j.jhazmat.2022.130321
Yan, Z., Ji, H., Guo, G., Xia, F., Zhang, W., Song, S., & Liu, S. (2023). Geochemical characteristics of trace elements and REEs and their geological significance for uranium mineralization within the Qianjiadian Sandstone-Hosted uranium deposit, Songliao Basin. Geofluids, 2023. https://doi.org/10.1155/2023/6297033
Yang, X., Liu, J., McGrouther, K., Huang, H., Lu, K., Guo, X., ... & Wang, H. (2016). Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Environmental Science and Pollution Research, 23, 974-984. https://doi.org/10.1007/s11356-015-4233-0
Yang, J., Guo, Z., Jiang, L., Sarkodie, E. K., Li, K., Shi, J., ... & Liu, X. (2022). Cadmium, lead and arsenic contamination in an abandoned nonferrous metal smelting site in southern China: Chemical speciation and mobility. Ecotoxicology and Environmental Safety, 239, 113617. https://doi.org/10.1016/j.ecoenv.2022.113617
Yang, Y., Zhang, L., Huang, X., Zhou, Y., Quan, Q., Li, Y., & Zhu, X. (2020). Response of photosynthesis to different concentrations of heavy metals in Davidia involucrata. PLoS One, 15(3), e0228563. https://doi.org/10.1371/journal.pone.0228563
Younis, U., Malik, S. A., Rizwan, M., Qayyum, M. F., Ok, Y. S., Shah, M. H. R., ... & Ahmad, N. (2016). Biochar enhances the cadmium tolerance in spinach (Spinacia oleracea) through modification of Cd uptake and physiological and biochemical attributes. Environmental Science and Pollution Research, 23, 21385-21394. https://doi.org/10.1007/s11356-016-7344-3
Zarcinas, B. A., McLaughlin, M. J., & Smart, M. K. (1996). The effect of acid digestion technique on the performance of nebulization systems used in inductively coupled plasma spectrometry. Communications in Soil Science and Plant Analysis, 27(5–8), 1331–1354. https://doi.org/10.1080/00103629609369636
Zhang, N., Xing-Dong, H. E., Yu-Bao, G. A. O., Yong-Hong, L. I., Hai-Tao, W. A. N. G., Di, M. A., ... & Yang, S. (2010). Pedogenic carbonate and soil dehydrogenase activity in response to soil organic matter in Artemisia ordosica community. Pedosphere, 20(2), 229-235. https://doi.org/10.1016/S1002-0160(10)60010-0
Zhao, Y., & Naeth, M. A. (2022). Lignite derived humic products and cattle manure biochar are effective soil amendments in cadmium contaminated and uncontaminated soils. Environmental Advances, 8, 100186. https://doi.org/10.1016/j.envadv.2022.100186
Zhou, Z., Xu, Z., Feng, Q., Yao, D., Yu, J., Wang, D., ... & Zhong, M. E. (2018). Effect of pyrolysis condition on the adsorption mechanism of lead, cadmium and copper on tobacco stem biochar. Journal of Cleaner Production, 187, 996-1005. https://doi.org/10.1007/s11356-019-05917-1
Zhu, Y., Wang, H., Lv, X., Zhang, Y., & Wang, W. (2020). Effects of biochar and biofertilizer on cadmium-contaminated cotton growth and the antioxidative defense system. Scientific Reports, 10(1), 20112. https://doi.org/10.1038/s41598-020-77142-7
Ziadi, N., Gagnon, B., & Nyiraneza, J. (2013). Crop yield and soil fertility as affected by papermill biosolids and liming by-products. Canadian Journal of Soil Science, 93(3), 319–328. https://doi.org/10.4141/cjss2012-129
Zulqurnain Haider, M., Hussain, S., Muhammad Adnan Ramzani, P., Iqbal, M., Iqbal, M., Shahzad, T., ... & Mahmood, F. (2019). Bentonite and biochar mitigate Pb toxicity in Pisum sativum by reducing plant oxidative stress and Pb translocation. Plants, 8(12), 571. https://doi.org/10.3390/plants8120571
Acknowledgements
The authors would like to thank Assoc. Prof. Dr. Ali Rıza Ongun for his expert technical assistance during the analytical procedures, the EUAF Department of Crop Protection for their kind permission to grow the plants under controlled conditions in their greenhouse, and Mr. Robin Healey for revising the language of the initial draft of the manuscript.
The collection, analysis, and interpretation of data for this work are funded by Ege University Rectorship (project no. 16-ZRF-066). The writing of the report and the decision to submit the manuscript for publication were partly funded by the Czech grant agencies Czech National Agency for Agricultural Research and Technology Agency of the Czech Republic, with grant numbers QK 1910086 and TJ01000071, respectively.
Funding
Open access publishing supported by the National Technical Library in Prague. The collection, analysis, and interpretation of data for this work are funded by Ege University Rectorship (project no. 16-ZRF-066). The writing of the report and the decision to submit the article for publication were partly funded by the Czech grant agencies Czech National Agency for Agricultural Research and Technology Agency of the Czech Republic, with grant numbers QK 1910086 and TJ01000071, respectively.
Author information
Authors and Affiliations
Contributions
R. S. Kara: Conceptualization, Methodology, Investigation (ageing of the soils after artificial contamination, biochar production and incubation, analyses of soil and biochar), Formal analyses, Data curation, Writing - original draft preparation, Project administration. S. Pazarlar: Conceptualization, Methodology, Investigation (biochar production, plant growth, plant analyses), Formal analyses, Data curation, Writing - original draft preparation, Project administration. B. Okur: Writing - review and editing, Supervision (regarding soil analyses), Funding acquisition (Grant no. 16-06-066, Ege University Rectorship). C. Almaz: Writing - review and editing, Investigation (ageing of the soils after artificial contamination, biochar incubation, analyses of soils). N. Okur: Writing - review and editing, Supervision (regarding soil analyses). S. Matula: Writing - review and editing, Funding acquisition (Grant no. QK1910086, Czech National Agency for Agricultural Research). M. Miháliková: Writing - review and editing, Funding acquisition (Grant no. TJ01000071, Technology Agency of the Czech Republic).
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Publish
Not applicable.
Consent to Participate
Not applicable.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kara, R.S., Pazarlar, S., Okur, B. et al. Texture and Contamination-Level Dependent Effects of Calcium-Rich Deinking Paper Sludge Biochar on Soil Cd Availability, Enzymatic Activity, and Plant Stress Mitigation. Water Air Soil Pollut 235, 454 (2024). https://doi.org/10.1007/s11270-024-07202-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07202-5