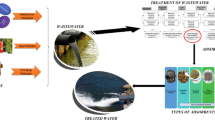
Abstract
Over a billion people in developing countries do not have access to clean water. Industries such as textiles, tanneries, and plastics release wastewater with toxic dyes that require treatment to remove the color. Adsorption and coagulation are known methods for removing dyes from wastewater. The production of natural adsorbents and coagulants involves the use of waste from agricultural products, which makes them cost-effective and environmentally friendly. They can be modified to achieve high dye removal rate. Natural adsorbents and coagulants are more eco-friendly than synthetic materials such as activated carbon and alum, because they can achieve similar levels of removal efficiency while being biodegradable and reusable. The combination of two methods can help to reduce the required dosage of adsorbent and coagulant and simultaneously improve the rate of dye removal. The purpose of this review is to evaluate and contrast the efficacy of recent natural agricultural adsorbents and coagulants, as they are environmentally friendly and abundant in nature. Additionally, it aims to introduce novelty by reviewing the combined coagulation/flocculation and adsorption systems in the treatment process for removing dyes, utilizing adsorbents and coagulants derived from agricultural wastes. As it turns out, the most examined dyes for removal were Methylene Blue and Congo Red and as adsorbents were used banana peels, orange peels, and nut. Furthermore, Moringa oleifera is used as a coagulant in both single and combined systems. Regarding adsorption, it was observed that banana peels could remove different dyes with high efficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
For humanity, water is essential. Mostly in developing countries, more than one billion people live without access to potable water (Onukwuli & Obiora-Okafo, 2019; Zhao et al., 2022). Nowadays, textile, tannery, paper, plastic, and paint are some of the industries that pollute the water. Wastewater from that industries contains toxic dyes if the necessary treatment is not applied to remove the color from the water (Dai et al., 2018; Gayathri Manju et al., 2019; Kholiya et al., 2021; Kim et al., 2019; Memon et al., 2021; Miao et al., 2022; Mohammadi et al., 2022; Pormazar & Dalvand, 2022; Thillainayagam et al., 2022; Wang et al., 2018). Ionic and non-ionic dyes are the two main types of dye. Dyes are also categorized according to their color and the functional group that is associated with their chemical structure. Ionic dyes are further subdivided into cationic and anionic dyes. Anionic dyes are classified as acid, direct, and reactive. Non-ionic dyes can be separated into Vat, dispersed, and solvent (Fobiri, 2022; Yeow et al., 2021).
Cationic dyes contain a positively charged ion (cation) and are commonly used in the textile, paper, and printing industries (Panda et al., 2021). These dyes are attracted to the negatively charged surfaces of materials, which allows them to bond strongly and create vibrant and long-lasting colors. However, cationic dyes are also known to be toxic and can be harmful to human health and the environment if they are not properly treated before being released into the environment (Kubra et al., 2021).
Acid dyes are water-soluble and are commonly used in the textile and leather industries to dye wool, silk, nylon, and other protein-based fibers. These dyes are acidic in nature and have a negatively charged ion that allows them to bond with the positively charged surfaces of the fibers. Acid dyes can create vibrant and long-lasting colors, but they are also known to be toxic and can be harmful to human health and the environment if they are not properly treated before being released into the environment (Rahdar et al., 2021).
Direct dyes are water-soluble and are commonly used in the textile industry to dye cotton, rayon, and other cellulose-based fibers. Direct dyes are so-called because they can be applied directly to the fibers without the need for a mordant or other chemical treatment. Direct dyes can create bright and vibrant colors, but they are also known to be less durable and more likely to fade than other types of dyes. Additionally, direct dyes can be harmful to the environment if they are not properly treated before being released into the environment (Mousavi et al., 2021).
Reactive dyes chemically react with the fibers that they are applied to, forming a covalent bond. These dyes are commonly used in the textile industry to dye cotton, rayon, and other cellulose-based fibers. Reactive dyes can create vibrant and long-lasting colors that are resistant to fading and washing, and they are also less toxic and more environmentally friendly than other types of dyes. Moreover, reactive dyes require a significant amount of water and energy to produce and can be difficult to remove from wastewater if not properly treated (Ahmed & Majewska-Nowak, 2020).
Vat dyes are insoluble in water and require a reducing agent, such as sodium hydrosulfite, to convert them into a soluble form before they can be applied to fibers. Vat dyes are commonly used in the textile industry to dye cotton, wool, and other natural fibers, and they can create deep and long-lasting colors that are resistant to fading and washing. Vat dyes are also known to be more environmentally friendly than other types of dyes, as they are less likely to leach into the environment and can be easily removed from wastewater with proper treatment (Fobiri, 2022).
Disperse dyes are commonly used to color synthetic fibers, such as polyester, nylon, and acetate (Panda et al., 2021). Disperse dyes are so-called because they are dispersed in water with the help of a dispersing agent before being applied to the fibers. Disperse dyes can create bright and vibrant colors that are resistant to fading and washing, but they are also known to be removed difficult from wastewaters (Mahmoudabadi et al., 2019).
Solvent dyes are soluble inorganic solvents, such as acetone, ethanol, and chloroform. Solvent dyes are commonly used in the plastic, ink, and automotive industries to color a wide range of materials, including plastics, waxes, and oils. Solvent dyes can create bright and vibrant colors that are resistant to fading and have good light-fastness and weather-resistance properties. However, solvent dyes are also known to be harmful to human health and the environment (Rane & Joshi, 2021).
In Table 1 is tabulated the classification of synthetic dyes and some examples with the relative wavelengths of their absorption in UV–Vis spectroscopy.
Different physicochemical procedures, such as adsorption, coagulation/flocculation, oxidation and ozonation, membrane separation, ion exchange, anaerobic decolorization, and reverse osmosis, used to remove color from effluents (Sukla Baidya & Kumar, 2021).
Adsorption refers to the process of adhesion of molecules or particles onto the surface of a solid or liquid. This process can have advantages and disadvantages. On the one hand, adsorption can be used to remove impurities and contaminants from fluids, gases, or solids. This is because the adsorbent material can selectively remove certain molecules or particles based on their size, polarity, or other properties. It can also be used to catalyze chemical reactions. In addition, adsorption can be expensive, especially when it involves the use of specialized materials or equipment. This can make it less accessible for certain applications or industries. Adsorbents have a finite capacity for adsorbing molecules or particles. Once the adsorbent is saturated, it needs to be replaced or regenerated before it can be used again. This can be a disadvantage in applications where continuous operation is required. Adsorption processes can produce waste or byproducts that can be harmful to the environment if not properly disposed or treated (Panda et al., 2021).
Coagulation/flocculation is a process that involves adding a coagulant to a liquid to destabilize the suspended particles and form larger flocs that can be more easily removed by sedimentation. Coagulation/flocculation removes suspended solids; it improves water quality by removing impurities like organic matter, bacteria, and viruses (Nnaji et al., 2022). In some cases, coagulation/flocculation can reduce the amount of chemicals needed for treatment, such as in cases where the coagulant can also act as a disinfectant. Otherwise, coagulation/flocculation can be an expensive process, and the flocs formed during coagulation are typically removed as sludge, which can be difficult and expensive to dispose of properly (Bui et al., 2016).
As an alternative to currently known physicochemical approaches, biodegradation technologies such as fungal decolorization, microbial degradation, adsorption by microbial biomass (alive or dead), and bioremediation systems have attracted attention (Niero et al., 2019).
In this research, comprehensive information is provided on the adsorbents and coagulants derived from agricultural wastes, along with detailed description of the experimental procedures employed for the removal of different type of dyes. Moreover, the objective is to present the combination of coagulation/flocculation and adsorption systems within the wastewater treatment process for dye removal, with the aim of using more economical and environmentally friendly materials. In this way, agricultural wastes are used and not discarded directly.
2 Adsorption
Adsorption is a physical technique that can effectively remove entire molecules from water. This process involves the uptake of the molecule from an aqueous solution onto an adsorbent material. The adsorbate can then be treated or removed in a separate environment, leaving the water stream free from any degraded products. A variety of materials have been developed and used as adsorbents for removing dyes. These materials include inorganic minerals, organic polymers, and carbon-based materials such as activated carbon, graphene, biochar, and biomass-derived carbon material, as well as biomasses like cellulose and chitosan (Kholiya et al., 2021; Miao et al., 2022; Zazycki et al., 2018; Zhang et al., 2018) and other modified nanomaterials (Rahdar et al., 2021). Moreover, activated carbon, commonly used for removing pollutants from air and water, was one of the best adsorbents for dye removal. However, the main disadvantage of activated carbon as an adsorbent is its relatively high cost and the difficulty in regenerating it once it becomes saturated with adsorbate (Memon et al., 2021).
To address this issue, researchers have explored the use of natural materials as low-cost adsorbents. These materials can be easily obtained, and their use as adsorbents can help to reduce the overall cost of the process while also providing an environmentally sustainable solution. To improve the efficiency of adsorption, natural waste materials can be chemically or physically modified (Sun et al., 2021). This modification process can alter the surface chemistry and physical properties of the material, making it more effective at adsorbing specific contaminants. For example, physical modification techniques such as grinding or crushing can increase the surface area of the adsorbent material, providing more active sites for adsorption. Chemical modification techniques such as acid treatment, oxidation, or grafting can introduce functional groups onto the surface of the adsorbent material, enhancing its ability to interact with specific contaminants. These modifications can improve the performance of natural waste materials as adsorbents and increase their potential for use in water treatment and other applications (Harnal et al., 2020; Mousavi et al., 2021; Ndagijimana et al., 2021; Suma et al., 2019). Some of the natural adsorbents, such as zeolites, MOF compounds, and natural polymers, are widely used in literature for dye removal. Moreover, it is not widely derived from agricultural wastes (Mohammadi et al., 2022), and for this reason are not included in this review.
Adsorption can be conducted in batch experiments and/or in column systems. In batch experiments, a certain dosage of adsorbent is mixed with dye solution, allowing them to interact over a specific period of time. However, column systems involve passing the dye solution through a packed column of adsorbent material, supporting a continuous or semi-continuous adsorption process. According to literature, column systems are rarely used for dye removal using natural agricultural materials, mainly due to concerns related to material availability and cost (Thillainayagam et al., 2022).
2.1 Activated Carbon from Agricultural Waste
According to Pourali et al. (Pourali et al., 2021), the adsorbent was derived from walnut peel and activated carbon. ZnO nanoparticles were the production of appropriate green processing of walnut peel. After that, ZnO particles mixed with activated carbon and AC-ZnO adsorbent composited to eliminate Acid Blue 113. The parameters of the experiment were pH, dosage of adsorbent, contact time when removal reached a plateau, and Acid Blue 113 concentration and the results were 3, 0.5 g/L, 15 min, and 100 mg/L, respectively. The peak removal rate of the dye was 98.2% with previous parameters at room temperature. The Langmuir model describes better the experiment of adsorption and the max adsorption capacity was 333.3 mg/g. It is noticeable that AC-ZnO with Acid Blue 113 had 5 adsorption retrieval cycles with 73.9% removal rate in the last cycle. The recovery was with sodium hydroxide. Finally, the experiment took place with a real sewage sample and reached 81.6% removal (Pourali et al., 2021).
Moreover, activated pine cone biochar treated with hydroxide potassium (APC) to be utilized as a modified natural absorbent (Kaya et al., 2022). The perfect removal (94.6%) of Congo Red 25 mg/L concentration achieved when pH value was 4 and 2.0 g/L was the dosage of APC. The maximum contact time was 1 h, and adsorption ability was 45.7 mg/g at 318 K. The Freundlich model was found to be the fittest for isotherm experiment and pseudo-second-order model for kinetic (Kaya et al., 2022).
As per Nizam et al. (Nizam et al., 2021), biomass of rubber seed and shell was modified to activated carbon with synthesis procedures such as acid and base impregnation, pyrolysis, washing and soaking, and finally grinding. The dyes studied were Congo Red and Methylene Blue and the removal rate was 100% approximately for each adsorbent. For the adsorption with activated carbon derived from rubber seed (RS) and shell (RSS) and Congo Red, pH, dosage, concentration, and contact time were 4, 0.9 g/L, 20 mg/L, and 10 min for each adsorbent, respectively. Otherwise, for Methylene Blue were 11, 40 mg/L, and 10 min for RS and 25 min for RSS, respectively. Mechanism of the adsorption was complicated. The Langmuir model was suitable for Congo Red, the Freundlich for Methylene Blue, and the pseudo-second-order kinetic mode for both. Max adsorption capacities were for Congo Red 227.27 mg/g by RS and 458.43 mg/g by RSS and for Methylene Blue were 769.2 mg/g (RS) and 659.35 mg/g (RSS). It is referred that ionic strength declined the removal of dyes. After 10 cycles of regeneration, it is observed a moderate fall in percentage of removal (Nizam et al., 2021).
Furthermore, Ndagijimana et al. (Ndagijimana et al., 2021) composited a new natural adsorbent from cassava flour powder and granula activated carbon and graphene oxide, PAC-GO and GAC-GO, respectively. PAC-GO and GAC-GO prepared with the same way. Cassava flour diluted with ultrapure water and filtered to produce cassava extract solution (CES), cross-linked activated carbon and graphene oxide with CES, and were sintered to produce them. Both were removed Direct Red 23 and Methylene Blue dyes. The best conditions of experiments for 30% and 40% Direct Red 23 removal rate by GAC-CO and PAC-GO respectively were at pH 3, dosage of each adsorbent 2.0 g/L, initial concentration 20 mg/L for the former, and 100 mg/L for the latter. Contact time until the equilibrium was 180 min for GAC-CO and 120 min for PAC-GO. However, purification of Methylene Blue just under 100% achieved at 100 mg/L concentration and 0.5 g/L adsorbents. pH of dye negligible influenced the removal. For the best elimination of Methylene Blue needed 5 min for both adsorbent. The Langmuir isotherm model fitted to that dyes adsorption and followed pseudo-second order model. The biggest adsorption capacity for Reactive Red 23 was 66.8 mg/g GAC-GO and 114.8 mg/g PAC-GO, and for Methylene Blue 222.7 mg/g GAC-GO and 248.1 mg/g PAC-GO (Ndagijimana et al., 2021).
Banana peel was modified with FeSO4 to remove Methylene Blue by Zhang et al. (Zhang et al., 2020). Banana peel extract derived from dust of banana peel with distilled water after filtration and added FeSO4 under ultrasonic energy to prepare the adsorbent (banana biochar/FeSO4). For 500 mg/L concentration of dye needed 2.0 g/L of adsorbent at pH 6.1, 313 K to succeed the highest adsorption capacity 862.0 mg/g. Both the pseudo-second-order model and the Langmuir model successfully explain the kinetic and isotherm analysis. The new adsorbent had five times reusability with good decolorization (Zhang et al., 2020).
In the study of Sun et al. (Sun et al., 2021), Methylene Blue in 150 mg/L initial concentration decreased by 99.52% by 0.3 g/L of activated carbon from Corncob (CCAC) at pH 5. The stages that corncob became CCAC were pre-carbonization protected by N2, mixture with potassium hydroxide, heat, washing with sulfuric acid, and deionized water until pH 7. Adsorption demanded 360 min to achieve the equilibrium and the Langmuir model fitted good at isotherm with biggest capacity being 864.6 mg/g at 298 K. The pseudo-second-order model lined with the kinetic study. After 5 cycles of experiment, elimination rate of Methylene Blue remained at 99.3% (Sun et al., 2021).
Suma et al. (Suma et al., 2019) modified elephant (Elephas maximus) dung to activated carbon with sulfuric acid. The new natural modified elephant dung activated carbon (EDAC) studied to clean Methylene Blue from water. The batch experiment started without change of pH, 40 mg/L of Methylene Blue, and 2.0 g/L of EDAC to remove 100% of dye. The best removal accomplished in 120 min. The Langmuir model well-described the adsorption of EDAC with the highest adsorption capacity being 19.0 mg/g (Suma et al., 2019).
Activated carbon from fruit peel, Hydnocarpus pentandra, was studied by Nayak et al. (Nayak et al., 2020). The production of activated carbon was with ZnCl2 treatment. A successful percentage of removal of 88.6% of Methylene Blue solution accomplished with 250 mg/L dye, 1.0 g/L activated carbon dose at pH between 6 and 7, 328 K. For the adsorption equilibrium needed 280 min (Nayak et al., 2020).
Regarding the study of Sawalha et al. (Sawalha et al., 2022), plenty of waste of biomass (coffee grains, date pits, jute and grapevine sticks, almond, pistachio, peanut, and sunflower shells) could be used both as natural absorbents or as modified activated carbons. The research showed that all adsorbents had reduced Methylene Blue specifically from an aqueous solution, but the best was the activated carbon from sunflower shells. The preparation of activated carbon started with heat of ZnCl2 and sunflower mixture and finished with pyrolysis of it. The new activated carbon at 0.5 g/L dosage realized just over 99.0% of 50 mg/L dye removal at pH 7, 298 K. The duration of the experiment was 24 h. The model that applied to the isotherm study was the Langmuir with the highest level of adsorption capacity being attained at 29.8 mg/g (Sawalha et al., 2022).
Another activated carbon derived from fruit is GWAC (grape wood activated carbon), made by Mousavi et al. (Mousavi et al., 2021). Grape wood waste sieved, washed, and treated with sulfuric acid and after that produced GWAC. 96.8% of 100 mg/L of Reactive Red 2 removed from water when pH was 3 and added 122.5 g/L GWAC. The time that needed to reach a plateau was 90 min, the adsorption followed the Langmuir (type II) isotherm model with the maximum adsorption capacity being 454.5 mg/g and expressed better from pseudo-second-order (type II) model (Mousavi et al., 2021).
As stated by Zazycki et al. (Zazycki et al., 2018), when pecan nutshell pyrolyzed, a biochar was produced and 85% elimination of Reactive Red 141 dye solution was achieved. To be reached this removal, the initial dye concentration was 50 mg/L and 1.0 g/L biochar dosage adjusted to pH 3. Equilibrium was succeeded after 10 min. The models which well-depicted were the Freundlich isotherm and pseudo-second order (Zazycki et al., 2018).
The Remazol Brilliant Blue Reactive dye was decolorized using modified black walnut shell biomass that had been treated with nitric acid to produce activated carbon black walnut shell (WSBAC) (Parimelazhagan et al., 2022). To remove 98.2% of it, the ideal conditions were 7.0 g/L WSBAC and 150 mg/L of dye at pH 1.5 in 24 h. Langmuir and pseudo-second-order model were shown to be more applicable for isotherm and kinetic studies. The greatest adsorption ability was 54.4 mg/g. This new adsorbent was able to be reused for three times (Parimelazhagan et al., 2022).
Table 2 summarizes all the conditions which took place for each adsorption experiment and categorized with the dye. The remarkable is that anionic colors, as Acid Blue 113, Congo Red, Direct 23, Reactive Red 2,141, and Remazol Brilliant Blue Reactive, were needed low pH values for their removal. Otherwise, Methylene Blue, a cationic dye, requires high pH value except for CCAC and banana biochar. The new activated carbons that derived from natural products had high adsorption capacity with the maximum being 864.6 mg/g of CCAC (Zhang et al., 2020). These methods shown that new materials had a good reusability and consider them as low budget adsorbent (Kaya et al., 2022; Mousavi et al., 2021; Nayak et al., 2020; Ndagijimana et al., 2021; Nizam et al., 2021; Parimelazhagan et al., 2022; Pourali et al., 2021; Sawalha et al., 2022; Suma et al., 2019; Sun et al., 2021; Zazycki et al., 2018; Zhang et al., 2020). Figure 1 compares the activated carbon biochar from agricultural products according the percentage of removal. As it turns out, most materials remove dyes at high rates, approaching 100%. The exception is GAC-GO and PAC-GO adsorbents examined for Direct Red 23 presenting only 30–40% removal.
2.2 Natural Agricultural Adsorbents
Argan nutshell wood (ANW) was tested as a new adsorbent. By applying 24.0 g/L of ANW 91.5% removal of 100 mg/L Congo Red dye solution, achieved at pH 6.08. The pseudo-second-order model suited more to the kinetic mechanism and the contact time to attain the equilibrium was 1 h. The Langmuir model expressed the isotherm mechanism and highest adsorption ability was 12.24 mg/g. The remarkable of this study is that ANW used to remove two binary systems with Methylene Blue and Crystal Violent. The experiment took place like Congo Red alone, but the ratios of the dyes were (2:8) Congo Red-Methylene Blue and Congo Red-Crystal Violet and observed 12.24 mg/g and 12.06 mg/g, respectively (El Khomri et al., 2022).
Banana peel reduced 75.3% of 20 mg/L Congo Red aqueous solution at pH 10 with the dosage of natural adsorbent being 18.8 g/L. Kinetic experiment showed the best model was pseudo-second order and equilibrium needed 90 min to realized. The isotherm data presented better by the Langmuir model and the best capacity was 1.727 mg/g at 313 K. 1 N NaOH used for desorption and the recovery of the dye was 97.3% (Mondal & Kar, 2018).
One other aspect of Congo Red removal was from Zewde et al. (Zewde & Geremew, 2022) but with natural coagulant being Vernonia amygdalina leaf powder (VALP). VALP used a dosage of 3.3 g/L to reduce 75.3% of 100 mg/L aqueous dye solution at pH 8 with a contact time being 70 min. The Langmuir and pseudo-second order described as the best models for isotherm and kinetic study, respectively. The maximum adsorption capacity calculated at 57.47 mg/g (Zewde & Geremew, 2022).
In the paper published by Ahmed et al. (Ahmed et al., 2020), 2.0 g/L of orange peel dust adsorbed 86.7% of 50 mg/L Crystal Violet in water solution at pH 8, 303 K. The Langmuir model was the best model for isotherm study and Qmax was 138.9 mg/g. Kinetic mechanism presented better by pseudo-second-order model and contact time was 70 min. Regeneration applied for five times with HCl as eluent and the removal was at the same level (Ahmed et al., 2020).
Over and above, two different types of adsorbents were banana peel and sugarcane bagasse. Experiments indicated 1.0 g/L of each adsorbent, at neutral pH, used to remove 100 mg/L Eurozol Navy Blue percentage of 72.0% and 70.0%, respectively. Isotherm experiment provided the Langmuir model was the best for both and Qmax was 24.1 mg/g (banana peel) and 32.5 mg/g (sugarcane bagasse). The equilibrium was accomplished in 1 h. The research was continued by blending adsorbents and under the same conditions, the results were 68.0% dye removal and 27.5 mg/g the maximum adsorption capacity (Ahmed & Majewska-Nowak, 2020).
Fadhil et al. (Fadhil et al., 2021) conducted interest research about adsorption of Indigo Red on corn leaves. The batch adsorption experiment lasted 4 days with 10 mg/L Indigo Red, 0.25 g/L corn leaves dose at pH 12, 303 K and removed 91.0% of dye. The Freundlich and pseudo-second-order models fitted with isotherm and kinetic studies (Fadhil et al., 2021).
Moreover, orange and banana peels were studied for a different dye removal by Maheshwari et al. (Maheshwari et al., 2022). In this case, Malachite Green (100 mg/L) had 72.0% of the dye removed with 12 g/L banana peel and 70.0% with 14.0 g/L orange peel in 100 min, at pH 8 and 303 K. Maximum adsorption capacity calculated at 41.0 mg/g and 42.6 mg/g, respectively. The Langmuir model beseemed well to the isotherm study (Maheshwari et al., 2022).
Banana peel, also, was supported by Dey et al. as an effective adsorbent. Betel nut husk was efficient too in Methylene Blue decolorization (Dey et al., 2017). It was pointed out that 50 mg/L of dye fall by 94.8% and 91.6% for 20 g/L banana and betel nut, respectively. pH had no impact in this adsorption study. Both Langmuir and Freundlich models befitted in the isotherm mechanisms for banana, but for betel nut suited Temkin model. Adsorption capacity calculated for banana at 23.9 mg/g and 0.9 mg/g for betel nut. The pseudo-second-order model well-satisfied the kinetic study (Dey et al., 2017).
Banana leaf was heated to produce new bio-adsorbent (BLA) to decline Methylene Blue concentration from water (Alam et al., 2022). Optimum conditions were pH 8.7, 90 mg/L dye, 0.239 g/L of 0.053–0.075 mm BLA, and 3 h to accomplish 93.8% (185.6 mg/g) removal at 303 K (Alam et al., 2022).
Cassava is a tropical plant and Chairunnisa et al. (Chairunnisa, 2020) used it for novel adsorbents. They created two adsorbents, cassava leaf power (CLP) with and without washing. CLP with washing (Qmax = 243.9 mg/g) adsorbed Methylene Blue better than without (Qmax = 178.5 mg/g). Initial dye concentration was 50 mg/L, adsorbent dose was 10.0 g/L, and pH value was 2 to examine the best dye removal. From kinetic study, contact time found to be 30 min and pseudo-second model to be the most suitable. From isotherm study, the Langmuir model mentioned as the best (Chairunnisa, 2020).
Peels from two fruits, mangosteen and pomegranate, were utilized as adsorbents to remove Methylene Blue dye by Phawachalotorn et al. (Phawachalotorn & Suwanpayak, 2021) and Daud et al. (Daud et al., 2019), respectively. The former reached 99.7% removal of 50 mg/L dye with 5.0 g/L of adsorbent dose (under 75 μm), pH value did not affect the procedure, and the contact time was 10 min. The latter decolorized the 99.8% of 10 mg/L dye with 10.0 g/L adsorbent dosage in 30 min at pH 11. The mangosteen peel-Methylene Blue system was described quiet well by the Freundlich isotherm model and pomegranate peel-Methylene Blue system by the Langmuir isotherm and pseudo-second-order kinetic model. The adsorption efficiency maximized at 9.97 mg/g for mangosteen peel and 102.6 mg/g for pomegranate peel (Daud et al., 2019; Phawachalotorn & Suwanpayak, 2021).
In the work of Kubra et al. (Kubra et al., 2021), turmeric powder used to eliminate the initial concentration of polluted water by Methylene Blue. The result of the experiment showed that the maximum overall Methylene Blue dye elimination of 99.5% was achieved when 1.5 g/L turmeric powder was added to 5 mg/L Methylene Blue at pH 7. Moreover, the isotherm study indicated that the Langmuir model was the most suitable and the highest adsorption capacity found to be 157.3 mg/g. The kinetic experiment showed that equilibrium was reached in 3 h. The turmeric power-Methylene Blue combination was reused up to seven times by ethanol as the eluent with approximately the same removal efficiency (Kubra et al., 2021).
In accordance with Krueger et al. (Krueger et al., 2019), watermelon and green coconut used as natural adsorbents. The efficacy of Remazol Brilliant Blue Reactive removal was over 80% for both materials under the most favorable conditions, which involved a dye concentration of 50 mg/L, an adsorbent dosage of 10.0 g/L, and a pH of 2. The study utilized the Freundlich isotherm model to represent the behavior of the watermelon and the Langmuir model to represent the behavior of the green coconut, as well as the pseudo-second-order model for both materials. The equilibrium achieved in 90 min and the maximum adsorption ability was 243.9 mg/g for watermelon and 2.2 mg/g for green coconut (Krueger et al., 2019).
Pomegranate peel dust was examined by Saigl et al. (Saigl & Ahmed, 2021) to purify the Rhodamine B dye solution. The initial concentration of the dye (5 mg/L) was decreased at 98.2% by 20 g/L amount of adsorbent at pH 5. The process of adsorption attained equilibrium after 10 min. The adsorption process befitted good with Langmuir isotherm model and pseudo-second-order kinetic model. The peak adsorption ability was found 31.95 mg/g (Saigl & Ahmed, 2021).
Comparing all the data presented in Table 3 and in Fig. 2, natural adsorbents decolorized efficiency the dyes from synthetic solutions. Banana and orange used in many studies with a great efficiency. It is worthy that no chemical modification done in the adsorbents. The highest adsorption capacity observed at 243.9 mg/g for CLP after washing and watermelon. As a result, all of these natural adsorbents are highly cost-effective and environmental-friendly for use in absorbing various substances (Dey et al., 2017; Mondal & Kar, 2018; Daud et al., 2019; Krueger et al., 2019; Ahmed & Majewska-Nowak, 2020; Chairunnisa, 2020; Ahmed et al., 2020; Phawachalotorn & Suwanpayak, 2021; Saigl & Ahmed, 2021; Fadhil et al., 2021; Kubra et al., 2021; Maheshwari et al., 2022; Zewde & Geremew, 2022; Alam et al., 2022; El Khomri et al., 2022). The removal rates, in all cases presented, were more than 70%.
2.3 Chemical Modified Natural Agricultural Adsorbents
Oak waste with base (NaOH), acid (HCl) chloroform and ethanol treatment prepared by Samarbaf et al.(Samarbaf et al., 2019) to deteriorate Acid Red 73 and Methylene Blue from water. It referred that 2.0 g/L of modified oak waste (MOW) succeeded 41.27% of reduction of 70.0 mg/L Acid Red 73 at pH 6.2 and 85.4% of the same concentration of Methylene Blue at pH 6.2 and needed 160 min for both. The adsorption capacity that provided was 64.8 mg/g for Acid Red 73 and 196.5 mg/g for Methylene Blue. In this study, the aim was to compare the four modifiers. It was found to be NaOH the best, HCl least adequate, ethanol, and finally the less effective was the chloroform (Samarbaf et al., 2019).
Raw wheat bran (WB) treated with cationic surfactants and multiple quaternary ammonium salts (MQAS) and MQAS-WB came of Zhang et al. (2018). It is proposed that the elimination of Acid Red 18 to be at pH 3, with initial dye concentration 50 mg/L and 1.0 g/L of adsorbent dose at 303 K. 98.9% removal of dye was achieved in 12 h. The biggest capacity of adsorption was 65.4 mg/g. Isotherm study was finest expressed by the Langmuir model and kinetic study by pseudo-second-order model (Zhang et al., 2018).
Additionally, areca nut husk is another fruit that has been modified by sodium hydroxide and by which 97% purification of Brilliant Green dye observed. The ideal conditions were at pH 7, 100 mg/L of the dye, and 10.0 g/L of areca nut at 298 K. It was needed only 120 min to stabilize the removal at 97%. The pseudo-second-order and Langmuir models were suitable for kinetic and isotherm studies, respectively. Isotherm diagram gave information about maximum adsorption capacity which was 18.2 mg/g (Sukla Baidya & Kumar, 2021).
Magnetized orange peel (MOP) used to decolorize Crystal Violet dye from aqueous solution (Ahmed et al., 2020). Orange peel powder was mixed with iron(III) chloride (FeCl3), iron(II) chloride dihydrate (FeCl2∙H2O), and double deionized water, then iron(II, III) oxide (Fe3O4) was precipitated with ammonia, ammonium hydroxide was added to control the pH, and a magnet was used to separate MOP. It succeeded 91.1% of removal when in 50 mg/L concentration of Crystal Violet added 1.0 g/L of MOP at pH 8, 303 K. The maximum adsorption capacity was 555.6 mg/g and the Langmuir model was the best. Contact time was found to be 70 min and pseudo-second order described well the kinetic mechanism. It is noticeable that MOP had 5 cycles regeneration with hydrochloric acid (Ahmed et al., 2020).
The surface of Casuarina equisetifolia pine powder was modified with H2SO4 (SFCE) by Chandarana et al. for decolorizing polluted water with Methylene Blue (Chandarana et al., 2020). The best experimental process for 99.0% removal was when 0.5 g/L SFCE was added into 100 mg/L initial dye concentration in 1 h at pH neutral and 323 K. The mechanism of chemical adsorption was observed by pseudo-second model. The Freundlich model fitted perfectly in an isothermal study with a maximum adsorption efficiency of 42.2 mg/g (Chandarana et al., 2020).
Another modification of orange was expressed by Munagapati et al. and it was quaternary amine-modified orange peel powder (QAMOPP) (Munagapati et al., 2019). For Reactive Red 120 (70 mg/L), 91% of decolorization reached with the QAMOPP (2 g/L) at pH 2. It is believed that two models fitted to the isotherm analysis, Langmuir and Khan, with the best being the Langmuir (R2 = 0.9996) and the highest adsorption capacity 344.8 mg/g. At kinetic experiment found that contact time was 110 min to reach the equilibrium and pseudo-second-order model was suitably. In the desorption study, 92.0% of dye recovered when NaOH utilized (Munagapati et al., 2019).
Other Reactive Red dye solution, Reactive Red 195, was purified by jute fiber modified by NaOH (NTJF). NaOH used wide to modify cellulose surface which jute fiber contains in its majority, as Dey et al. explain (Dey & Dey, 2021). The experimental parameters were pH 7, 14.0 g/L NTJF dosage, and 50 mg/L color concentration to reach almost the 100% of purification. From kinetic test, after 2 h, time was independent on the adsorption and pseudo-second-order model fit better. The Langmuir model was the most appropriate with Qmax = 32.2 mg/g at 303 K (Dey & Dey, 2021).
In short, Table 4 contains all the experimental information about the chemical modified adsorbents. Most of the adsorbents decolorized well the dye solutions. Furthermore, a lesser amount of the substance is needed to achieve effective adsorption. All the adsorption studies were followed by pseudo-second-order model that means chemisorption of solutes took place. The Langmuir model, which fitted in all adsorptions experiments except SFCE, assumes that adsorption occurred on a homogeneous surface and all adsorption sites are energetically identical. The Freundlich model depicts that adsorption sites are energetically independent of each other, and that adsorption is a multilayer process. The biggest adsorption capacity had MOP onto Crystal Violet dye, 555.6 mg/g and thus, MOP could be reused up to 10 times (Ahmed et al., 2020). These chemical modifications made adsorption more efficient and equilibrium reached faster (Ahmed et al., 2020; Samarbaf et al., 2019; Sukla Baidya & Kumar, 2021; Zhang et al., 2018). Figure 3 shows the comparison of removal efficiency (%) among chemically modified agricultural materials. Except Acid Red 73 removal by MOW (41%), the removal rates where > 90% for all modified natural agricultural adsorbents presented.
3 Coagulation/Flocculation
Coagulation/flocculation method is frequently utilized in the sewage treatment process, treating high strength industrial wastewaters (Tolkou & Zouboulis, 2020), such as dyes from effluent. Coagulation is a physico-chemical method that involves neutralizing the charge of colloidal pollution particles and then removing the pollutants by settling down the agglomerates that were formed. Chemical coagulation/flocculation is frequently used while electro-coagulation and biocoagulation are newer approaches. Coagulation/flocculation is less costly, easier to use, and capable of handling high initial concentrations (Chethana et al., 2016; Mahmoudabadi et al., 2019; Vijayaraghavan & Shanthakumar, 2016).
Coagulation/flocculation is a method that typically uses inorganic metals or organic polymers as chemical coagulants. Inorganic metals are alum, ferric and magnesium salts, lime etc. Organic polymers are polyacrylamide, polydiallyl-dimethyl-ammonium chloride etc. (Bui et al., 2016; Chethana et al., 2016; El Gaayda et al., 2022; Hoong & Ismail, 2018; Onukwuli & Obiora-Okafo, 2019; Ramesh et al., 2021; Vijayaraghavan & Shanthakumar, 2016). The selection of coagulant is critical in the removal of turbidity and color from wastewater. Although these coagulants are good at treating wastewater, they still have number of disadvantages associated with their usage. Some of them are ineffective at low temperatures, have relatively high supply costs, have the potential to cause human disease, are difficult to remove significant amounts of thickening sludge produced during their use, and produce treated water with extremely high or low pH levels. Alum has been the most widely used coagulant for several years, even though it causes Alzheimer’s and other neurodegenerative diseases in humans (Ali & El-Mohamedy, 2016; Bui et al., 2016; Chethana et al., 2016; El Gaayda et al., 2022; Hoong & Ismail, 2018; Jaeel & Ali, 2018; Onukwuli & Obiora-Okafo, 2019; Ramesh et al., 2021; Vijayaraghavan & Shanthakumar, 2016). Because of the limitations of the chemical coagulants, it is vital the progress in eco-friendly coagulants for the dye removal. These may derive from plants, animals, microorganisms, and the so-called natural coagulants (Ali & El-Mohamedy, 2016; Bui et al., 2016; Chethana et al., 2016; El Gaayda et al., 2022; Hoong & Ismail, 2018; Jaeel & Ali, 2018; Kristanda et al., 2021; Mahmoudabadi et al., 2019; Onukwuli & Obiora-Okafo, 2019; Ramesh et al., 2021; Vijayaraghavan & Shanthakumar, 2016).
3.1 Natural Agricultural Coagulants
Research from Onukwuli et al. (Onukwuli & Obiora-Okafo, 2019) deals with the removal of Alizarin Red and Naphthalene Black with tiger nut as coagulant. The coagulation parameters were pH value at 2, 0.6 g/L dose of tiger nut, 50 mg/L initial concentration of each dye, fast mixing was for 5 min at 100 rpm, slow for 30 min at 50 rpm, and rest for 30 min at 303 K. The purification of the color was 96.6% of Alizarin Red and 94.4% of Naphthalene Black (Onukwuli & Obiora-Okafo, 2019).
Additionally, Moringa oleifera (MO) seed used as natural coagulant by Gaayda et al. (El Gaayda et al., 2022) and Hadadi et al. (Hadadi et al., 2022). The first one used MO powder (MOSP) to remove Amino Black 10B color and the second one aqueous extract of MO (MOPW) and saline extract of MO (MOPS) for Mordant Black 11 (El Gaayda et al., 2022; Hadadi et al., 2022). MOSP reduced the 92.2% of 100 mg/L Amino Black 1OB when pH was 7 and 0.2 g/L MOSP was added. The mixing conditions were first at 120 rpm for 3 min, followed by 40 rpm at 20 min and were sedimented for 60 min (El Gaayda et al., 2022). MORW and MOPS achieved (80.12% and 95.02%, respectively) decolorization of 100 mg/L dye with 0.1 g/L dose of each coagulant, at pH 6.5. The samples were stirred for 3 min at 200 rpm, then 30 rpm for 20 min and immobilized for 60 min (Hadadi et al., 2022).
Furthermore, Acanthocereus tetragonus, Azadirachta indica, Cicer arietinum, and Moringa oleifera seeds surveyed as natural coagulants to minimize the Congo Red concentration by Chethana et al. (Chethana et al., 2016). In 500 mg/L dye at pH 3 adjusted 1.2 g/L of Acanthocereus tetragonus and reached 97.0% elimination. Azadirachta indica seed coagulant in 1.4 g/L amount mixed with the same Congo Red concentration to achieve 95% removal. Otherwise, only 0.8 g/L Cicer arietinum needed to fade the percentage of 89% of the dye. Finally, 1.5 g/L of Moringa oleifera seeds purified 500 mg/L Congo Red at pH between 3 and 5. The mixture was rapidly stirred at a speed of 200 rpm for a duration of 5 min, after which it was slowly stirred at 50 rpm for 15 min. It was then left to settle for a period of 1 h and 30 min (Chethana et al., 2016).
As stated by Kristanda et al. (Kristanda et al., 2021), the extract of Leucaena leucocephala seed successfully cleaned Congo Red dye by coagulation/flocculation. The protein from Leucaena leucocephala seeds (bio-coagulant) was obtained by extracting it with a solution of MgCl2. In order to eliminate 88.5% of the dye, a concentration of 44.1 mg/L of dye at pH 3.22 and 0.2 g/L of coagulant were required. Congo Red solution and leucine extract were combined and rapidly mixed at 200 rpm for 2 min, followed by slow mixing at 30 rpm for 30 min and sedimentation was achieved at 1-h rest (Kristanda et al., 2021).
Alcea rosea root mucilage referred as a new bio-coagulant by Mahmoudabadi et al. to minimize the final concentration of Disperse Red 60 and Reactive Blue 19 (Mahmoudabadi et al., 2019). Disperse Red 60 (40 mg/L) was 86.0% removed when pH was 11 and a 0.25 mg/L coagulant dose was added. On the other hand, Reactive Blue 19 had only 68% removal when 0.2 mg/L was adjusted to 20 mg/L dye concentration. Both were blended at 250 rpm for 2 min, followed by 60 rpm at 15 min, and finally 0 rpm for 60 min (Mahmoudabadi et al., 2019).
Papaya seed utilized as a new natural coagulant to remove 10 mg/L Drimaren Dark Red with 0.6 g/L dose at pH 1.97. The rapid mixing was for 1 min at 200 rpm, slow mixing for 30 min at 30 rpm, and sedimentation for 1 h to achieve 84.8% removal (Kristianto et al., 2018).
Another research by Bui et al. (Bui et al., 2016) endeavor applied Cassia fistula Linn seed gum to decrease Reactive Red 195 concentration. Adequate decolorization was achieved at 57.8% when 0.2 g/L coagulant added to 10 mg/L of dye at pH 10. The duration of slow mixing at 45 rotations per minute, which was the only mixing time provided, was 30 min. In the same experiment, Chemical Oxygen Demand (COD) had approximately equal extraction (Bui et al., 2016).
A novel coagulant, Capparis Spinosa, utilized to minimize a synthetic blue water solution (Jaeel & Ali, 2018). Coagulation/flocculation disappeared 96.0% of pigment, at pH 5.5 of 10 mg/L initial concentration with 0.1 g/L coagulant dosage. The speed of rapid stirring was 120 rpm for 2 min, then slow stirring was 2 rpm for 30 min and settled for 45 min to rest (Jaeel & Ali, 2018).
Table 5 summarizes the information provided regarding the process used to remove color from the solutions. This table likely includes details such as the initial concentration of each color in the solution, the pH range of the solution, and the dosage of the coagulant. The table may also include information on the efficiency of the process, such as the removal rate of each dye in the solution after treatment. Coagulants that mentioned above efficacy eliminate the dyes from the synthetic wastewater. Figure 4 depicts all natural coagulants than mentioned above compared the efficiency in coagulation/flocculation. As exhibit, the removal rates achieved by using natural coagulants derived from agricultural wastes are high, with the exceptional of Cassia fistula Linn. gum tested for Reactive Red 195 removal (58%).
4 Combined Systems
Recent researches have shown that combining multiple wastewater treatment processes into a single system is more effective than using individual processes (Lee et al., 2006). Nevertheless, the main disadvantage is that in general, combined processes are more complex than single processes and therefore require more specified equipment and personnel. Such a system is the adsorption-coagulation/flocculation hybrid process, which has been successfully used to treat wastewater from a complex dyes manufacturing plant and wastewater with high levels of humic and tannic acids. The hybrid process has been found to be 90.0% more effective at removing colloidal particles compared to using coagulation alone. In this process, coagulant and adsorbent are added simultaneously at one single unit for reaction. This approach improves the performance of dye removal by eliminating dye molecules from the water, rather than partially decomposing them, which could lead to the formation of more harmful and toxic aromatic compounds (Hoong & Ismail, 2018).
Hoong et al. (Hoong & Ismail, 2018) conducted research on a process that involves coagulation/flocculation followed by adsorption technique. Hibiscus sabdariffa seeds were used as a natural coagulant, while activated carbon served as the adsorbent. The optimal conditions for the process included an initial Congo Red concentration of 385 mg/L at pH 2, 0.23 mg/L coagulant dosage, and 0.15 g/L adsorbent dosage. The dye removal rate achieved under these parameters was as high as 96.7%. In the coagulation process, rapid mixing was at 100 rpm for 4 min, slow mixing at 40 rpm for 25 min, and settled for 30 min (Hoong & Ismail, 2018).
As previously reported, Morigna oleifera was an excellent natural coagulant (Chethana et al., 2016; El Gaayda et al., 2022; Hadadi et al., 2022). Ali et al. used it as coagulant but also used activated carbon as adsorbent (Ali & El-Mohamedy, 2016). Acid Red 27, Reactive Blue 19 and Reactive Blue 81 were the dyes that removed by 90.0% from their solutions. In order to achieve the removal of the colors, a starting solution was prepared with an initial concentration of 100 mg/L for each color, and a pH range of 8–10 was maintained. The solution was then treated with a coagulant at a concentration of 0.1 g/L, followed by adsorption with activated carbon at a concentration of 0.2 g/L. A rapid mixing step was carried out for 2 min at a rate of 100 rpm, followed by the slow mixing step for 30 min at a rate of 40 rpm. The time that the solution undergoes sedimentation was maintained at a steady 30 min throughout the process (Ali & El-Mohamedy, 2016).
To sum up, Table 6 contains all the information about the experimental process took place in combined systems. The minimum number of coagulants that were applied to purify high dye concentration of water solutions and the less activated carbon dosage used is noteworthy (Ali & El-Mohamedy, 2016; Hoong & Ismail, 2018).
5 Challenges and Opportunities
Natural agricultural adsorbents, as mentioned above, could be reused for adsorption after cleaning. Additionally, the challenge is to increase the number of reusability cycles while maintaining production costs (Nizam et al., 2021).
The struggle of using natural agricultural coagulants for dye removal is the proper disposal of the generated sludge. It is suggested that the dye-laden sludge could be dehydrated, dried, and subsequent be burned (Adegoke & Bello, 2015). Becoming a more popular disposal method involves utilizing natural agricultural adsorbents and/or coagulant sludge as fertilizers following chemical or biological treatment (Kar et al., 2022).
New research should emphasize the use of new natural agricultural adsorbents and coagulants for a combined system (coagulation/flocculation and adsorption) to minimize energy and costly expenditures associated with material production.
6 Conclusion
To summarize, adsorption and coagulation/flocculation are processes that have been used for many years. Natural agricultural adsorbents and natural agricultural coagulants have the advantages of being low-cost and environmentally friendly. They are also biodegradable and can be easily disposed of after use. However, their effectiveness can vary depending on the type and concentration of contaminants present in the water, and they may require pre-treatment or modification to improve their removal rates.
The most used dyes for removal were Methylene Blue and Congo Red and adsorbents were banana, orange, and nut. Moringa oleifera, also, used as a coagulant in the single and the combined system. In adsorption, it was observed that banana peel could remove different dyes with high efficiency. Furthermore, plenty of the novel adsorbents had a very good reusability. In coagulation/flocculation, Moringa oleifera compared with alum and the removal was at the same rate.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adegoke, K. A., & Bello, O. S. (2015). Dye sequestration using agricultural wastes as adsorbents. Water Resources and Industry, 12, 8–24.
Ahmed, A. E., & Majewska-Nowak, K. (2020). Removal of reactive dye from aqueous solutions using banana peel and sugarcane bagasse as biosorbents. Environment Protection Engineering, 46, 121–135. https://doi.org/10.37190/epe200308
Ahmed, M., Mashkoor, F., & Nasar, A. (2020) Development, characterization, and utilization of magnetized orange peel waste as a novel adsorbent for the confiscation of crystal violet dye from aqueous solution. Groundwater for Sustainable Development, 10, 100322. https://doi.org/10.1016/j.gsd.2019.100322
Ahsan, H., Shahid, M., Imran, M., et al. (2022). Photocatalysis and adsorption kinetics of azo dyes by nanoparticles of nickel oxide and copper oxide and their nanocomposite in an aqueous medium. PeerJ, 10, 1–26. https://doi.org/10.7717/peerj.14358
Alam, M. Z., Bari, M. N., & Kawsari, S. (2022). Statistical optimization of Methylene Blue dye removal from a synthetic textile wastewater using indigenous adsorbents. Environmental and Sustainability Indicators, 14, 100176. https://doi.org/10.1016/j.indic.2022.100176
Ali, N. F., & El-Mohamedy, R. S. R. (2016). Evaluation of Moringa oleifera seed extract coagulation in removal of some dyes in textile wastewater. International Journal of ChemTech Research, 9, 538–545.
Bui, H. M., Perng, Y., & Duong, H. (2016). The use of artificial neural network for modeling coagulation of reactive dye wastewater using Cassia fistula Linn. gum. Journal of Environmental Science and Management, 19, 1–8.
Chairunnisa, L. Y. (2020). Preliminary studies of cassava leaves’ ability to remove dyes from water. Journal of Mathematical and Fundamental Sciences, 52, 66–80. https://doi.org/10.5614/j.math.fund.sci.2020.52.1.5
Chandarana, H., Suganya, S., & Madhava, A. K. (2020). Surface functionalized Casuarina equisetifolia pine powder for the removal of hetero-polyaromatic dye: Characteristics and adsorption. International Journal of Environmental Analytical Chemistry. https://doi.org/10.1080/03067319.2020.1798418
Chethana, M., Sorokhaibam, L. G., Bhandari, V. M., et al. (2016). Green approach to dye wastewater treatment using biocoagulants. ACS Sustainable Chemistry & Engineering, 4, 2495–2507. https://doi.org/10.1021/acssuschemeng.5b01553
Dai, L., Zhu, W., He, L., et al. (2018). Calcium-rich biochar from crab shell: An unexpected super adsorbent for dye removal. Bioresource Technology, 267, 510–516. https://doi.org/10.1016/j.biortech.2018.07.090
Daud, N. D., Mahmad Puzi, S., Khalijah, S., & Rozi, M. (2019) New application of pomegranate peel waste: The decontamination of toxic Methylene Blue dye from textile wastewater. Journal of Engineering Research and Education, 11, 45–58.
de Krueger, M. D. S., Volkmann, A. C., & Rainert, K. T. (2019). Removal of textile dye Remazol Brilliant Blue Reactive (RBBR) using fibers of Citrullus lanatus (watermelon) and Cocos nucifera (green coconut) as adsorbent material. Revista Eletrônica em Gestão, Educação e Tecnologia Ambiental, 23, 5. https://doi.org/10.5902/2236117038526
Dey, A. K., & Dey, A. (2021). Selection of optimal processing condition during removal of Reactive Red 195 by NaOH treated jute fibre using adsorption. Groundwater for Sustainable Development, 12, 100522. https://doi.org/10.1016/j.gsd.2020.100522
Dey, M. D., Ahmed, M., Singh, R., et al. (2017). Utilization of two agrowastes for adsorption and removal of methylene blue: Kinetics and isotherm studies. Water Science and Technology, 75, 1138–1147. https://doi.org/10.2166/wst.2016.589
Dhaouadi, H., & M’Henni, F. (2009). Vat dye sorption onto crude dehydrated sewage sludge. Journal of Hazardous Materials, 164, 448–458. https://doi.org/10.1016/j.jhazmat.2008.08.029
El Khomri, M., El Messaoudi, N., Dbik, A., et al. (2022). Removal of Congo Red from aqueous solution in single and binary mixture systems using Argan nutshell wood. Pigment and Resin Technology, 51, 477–488. https://doi.org/10.1108/PRT-04-2021-0045
Fadhil, O. H. F. H., Eisa, M. Y., Salih, D. A., & Nafeaa, Z. R. (2021). Adsorption of Indigo Carmen dye by using corn leaves as natural adsorbent material. Al-Khwarizmi Engineering Journal, 17, 43–50. https://doi.org/10.22153/kej.2021.11.002
Fobiri, G. K. (2022). Synthetic dyeapplication in textiles: A review on the efficacies and toxicities involved. Textile & Leather Review, 5, 180–198. https://doi.org/10.31881/TLR
El Gaayda, J., Titchou, F. E., Barra, I., et al. (2022). Optimization of turbidity and dye removal from synthetic wastewater using response surface methodology: Effectiveness of Moringa oleifera seed powder as a green coagulant. Journal of Environmental Chemical Engineering, 10, 106988. https://doi.org/10.1016/j.jece.2021.106988
Gayathri Manju, B., Mathangi, J. B., Raji, P., & Helen Kalavathy, M. (2019). Equilibrium and kinetic studies on methylene blue adsorption by simple polyol assisted wet hydroxyl route of NiFe2O4nanoparticles 03 Chemical Sciences 0306 Physical Chemistry (incl. Structural). Journal of Environmental Health Science and Engineering, 17, 539–547. https://doi.org/10.1007/s40201-019-00368-9
Hadadi, A., Imessaoudene, A., Bollinger, J. C., et al. (2022). Parametrical study for the effective removal of Mordant Black 11 from synthetic solutions: Moringa oleifera seeds’ extracts versus alum, (Switzerland). Water, 14, 4109. https://doi.org/10.3390/w14244109
Harnal, V. S., Darla, U., & Lataye, D. H. (2020). Removal of Congo Red dye from wastewater using orange peel as an adsorbent. Journal of Indian Associations For Environmental Management, 40, 52–59. https://doi.org/10.56042/jiaem.v40i2.37674
Hoong H. N. J., & Ismail, N. (2018). Removal of dye in wastewater by adsorption-coagulation combined system with Hibiscus sabdariffa as the coagulant. MATEC Web Conferences, 152, 01008. https://doi.org/10.1051/matecconf/201815201008
Jaeel, A. J., & Ali, N. S. (2018). Color removal from textile wastewater using Capparis spinosa as natural coagulant. International Conference on Advance of Sustainable Engineering and its Application (ICASEA), 165–168. https://doi.org/10.1109/ICASEA.2018.8370976
Kar, S., Santra, B., Kumar, S., et al. (2022). Sustainable conversion of textile industry cotton waste into P-dopped biochar for removal of dyes from textile effluent and valorisation of spent biochar into soil conditioner towards circular economy. Environmental Pollution, 312, 120056. https://doi.org/10.1016/j.envpol.2022.120056
Kaya, N., Yıldız Uzun, Z., Altuncan, C., & Uzun, H. (2022). Adsorption of Congo Red from aqueous solution onto KOH-activated biochar produced via pyrolysis of pine cone and modeling of the process using artificial neural network. Biomass Conversion and Biorefinery, 12, 5293–5315. https://doi.org/10.1007/s13399-021-01856-5
Kholiya, F., Singh, A., Gosai, A., & Meena, R. (2021). Facile preparation of agaraldehyde chitosan-based composite beads as effectual adsorbent especially towards amido black. Journal of Applied Polymer Science, 138, 50716. https://doi.org/10.1002/app.50716
Kim, G. M., Wang, Z., Bin, Kang S., & Won, S. W. (2019). Polyethylenimine-crosslinked chitin flake as a biosorbent for removal of Acid Blue 25. Korean Journal of Chemical Engineering, 36, 1455–1465. https://doi.org/10.1007/s11814-019-0347-2
Kristanda, J., Sintiago, K. S., Kristianto, H., et al. (2021). Optimization study of Leucaena leucocephala seed extract as natural coagulant on decolorization of aqueous Congo Red solutions. Arabian Journal for Science and Engineering, 46, 6275–6286. https://doi.org/10.1007/s13369-020-05008-1
Kristianto, H., Angelina Kurniawan, M., & Soetedjo J. N. M. (2018). Utilization of papaya seeds as natural coagulant for synthetic textile coloring agent wastewater treatment. International Journal on Advanced Science, Engineering and Information Technology, 8, 2071–2077. https://doi.org/10.18517/ijaseit.8.5.3804
Kubra, K. T., Salman, M. S., & Hasan, M. N. (2021). Enhanced toxic dye removal from wastewater using biodegradable polymeric natural adsorbent. Journal of Molecular Liquids, 328, 115468. https://doi.org/10.1016/j.molliq.2021.115468
Lee, J. W., Choi, S. P., Thiruvenkatachari, R., et al. (2006). Evaluation of the performance of adsorption and coagulation processes for the maximum removal of reactive dyes. Dyes and Pigments, 69, 196–203. https://doi.org/10.1016/j.dyepig.2005.03.008
Lingeswari, U. D., & Vimala, T. (2020). Isotherm, kinetics and thermodynamic study of adsorption of phthalocyanine and azo dyes by CoCl2 doped polyaniline. Asian Journal of Chemistry, 32, 746–752. https://doi.org/10.14233/ajchem.2020.22411
Maheshwari, U., Thakur, R. V., Deshpande, D., & Ghodke, S. (2022). Efficiency evaluation of orange and banana peels for dye removal from synthetic industrial effluent. Materials-Today-Proceedings. https://doi.org/10.1016/j.matpr.2022.11.023
Mahmoudabadi, T. Z., Talebi, P., & Jalili, M. (2019). Removing Disperse Red 60 and Reactive Blue 19 dyes removal by using Alcea rosea root mucilage as a natural coagulant. AMB Express, 9, 113. https://doi.org/10.1186/s13568-019-0839-9
Memon, N., Kanwal, U., Memon, A., et al. (2021). Synthesis, characterization, and application of Co-Al-Zn layered double hydroxide/hydrochar composite for simultaneous removal of cationic and anionic dyes. Journal Chemistry, 2021, 1138493. https://doi.org/10.1155/2021/1138493
Miao, J. L., Ren, J. Q., Li, H. J., et al. (2022). Mesoporous crosslinked chitosan-activated clinoptilolite biocomposite for the removal of anionic and cationic dyes. Colloids and Surfaces B: Biointerfaces, 216, 112579. https://doi.org/10.1016/j.colsurfb.2022.112579
Mohammadi, A. A., Niazi, Z., Heidari, K., et al. (2022). Nickel and iron-based metal-organic frameworks for removal of organic and inorganic model contaminants. Environmental Research, 212, 113164. https://doi.org/10.1016/j.envres.2022.113164
Mondal, N. K., & Kar, S. (2018). Potentiality of banana peel for removal of Congo Red dye from aqueous solution: Isotherm, kinetics and thermodynamics studies. Applied Water Science, 8, 157. https://doi.org/10.1007/s13201-018-0811-x
Mousavi, S. A., Shahbazi, D., Mahmoudi, A., et al. (2021). Statistical modeling and kinetic studies on the adsorption of Reactive Red 2 by a low-cost adsorbent: Grape waste-based activated carbon using sulfuric acid activator-assisted thermal activation. Adsorption Science and Technology, 2021, 8404197. https://doi.org/10.1155/2021/8404197
Munagapati, V. S., Wen, J. C., Pan, C. L., et al. (2019). Enhanced adsorption performance of Reactive Red 120 azo dye from aqueous solution using quaternary amine modified orange peel powder. Journal of Molecular Liquids, 285, 375–385. https://doi.org/10.1016/j.molliq.2019.04.081
Nayak, S. S., Mirgane, N. A., Shivankar, V. S., et al. (2020). Adsorption of methylene blue dye over activated charcoal from the fruit peel of plant Hydnocarpus pentandra. Materials Today: Proceedings, 37, 2302–2305. https://doi.org/10.1016/J.MATPR.2020.07.728
Ndagijimana, P., Liu, X., Xu, Q., et al. (2021). Cassava flour extracts solution to induce gelatin cross-linked activated carbon-graphene oxide composites: The adsorption performance of dyes from aqueous media. Environmental Advances, 5, 100079. https://doi.org/10.1016/j.envadv.2021.100079
Niero, G., Corrêa, A. X. R., Trierweiler, G., et al. (2019). Using modified fish scale waste from Sardinella brasiliensis as a low-cost adsorbent to remove dyes from textile effluents. Journal of Environmental Science and Health. Part a, Toxic/hazardous Substances & Environmental Engineering, 54, 1083–1090. https://doi.org/10.1080/10934529.2019.1631091
Nizam, N. U. M., Hanafiah, M. M., Mahmoudi, E., et al. (2021). The removal of anionic and cationic dyes from an aqueous solution using biomass-based activated carbon. Scientific Reports, 11, 1–17. https://doi.org/10.1038/s41598-021-88084-z
Nnaji, P. C., Anadebe, V. C., Ezemagu, I. G., & Onukwuli, O. D. (2022). Potential of Luffa cylindrica seed as coagulation-flocculation (CF) agent for the treatment of dye wastewater: Kinetic, mass transfer, optimization and CF adsorption studies. Arabian Journal of Chemistry, 15, 103629. https://doi.org/10.1016/j.arabjc.2021.103629
Onukwuli, C. J., & Obiora-Okafo, I. A. (2019). Removal of dyes from synthetic waste water by coagulation technique using natural coagulant. SSRN Electronic Journal, 1–5. https://doi.org/10.2139/ssrn.3429569
Panda, S. K., Aggarwal, I., Kumar, H., et al. (2021). Magnetite nanoparticles as sorbents for dye removal: A review. Environmental Chemistry Letters, 19, 2487–2525.
Parimelazhagan, V., Yashwath, P., Arukkani Pushparajan, D., & Carpenter, J. (2022). Rapid removal of toxic Remazol Brilliant Blue-R dye from aqueous solutions using Juglans nigra shell biomass activated carbon as potential adsorbent: Optimization, isotherm, kinetic, and thermodynamic investigation. International Journal of Molecular Sciences, 23, 1–33. https://doi.org/10.3390/ijms232012484
Phawachalotorn, C., & Suwanpayak, N. (2021). The efficiency of mangosteen peel for dye removal. Journal of Physics: Conference Series, 1719, 1–8. https://doi.org/10.1088/1742-6596/1719/1/012061
Pormazar, S. M., & Dalvand, A. (2022). Adsorption of Reactive Black 5 azo dye from aqueous solution by using amine-functioned Fe3O4 nanoparticles with L-arginine: Process optimisation using RSM. International Journal of Environmental Analytical Chemistry, 102, 1764–1783. https://doi.org/10.1080/03067319.2020.1743278
Pourali, P., Behzad, M., Arfaeinia, H., et al. (2021). Removal of Acid Blue 113 from aqueous solutions using low-cost adsorbent: Adsorption isotherms, thermodynamics, kinetics and regeneration studies. Separation Science and Technology (philadelphia), 56, 3079–3091. https://doi.org/10.1080/01496395.2020.1867583
Rahdar, S., Rahdar, A., Sattari, M., et al. (2021). Barium/cobalt@polyethylene glycol nanocomposites for dye removal from aqueous solutions. Polymers (Basel), 13, 1161. https://doi.org/10.3390/polym13071161
Ramesh, S. S., Komala Devi, S., Tamilselvi, S., et al. (2021). Formulation of natural elite dye remover from textile effluent. International Journal of Current Research and Review, 13, 112–116. https://doi.org/10.31782/IJCRR.2021.13402
Rane, A., & Joshi, S. J. (2021). Biodecolorization and biodegradation of dyes: A review. Open Biotechnology Journal, 15, 97–108. https://doi.org/10.2174/1874070702115010097
Saigl, Z. M., & Ahmed, A. M. (2021). Separation of rhodamine b dye from aqueous media using natural pomegranate peels. Indonesian Journal of Chemistry, 21, 212–224. https://doi.org/10.22146/ijc.58592
Samarbaf, S., Tahmasebi Birgani, Y., Yazdani, M., & Babaei, A. A. (2019). A comparative removal of two dyes from aqueous solution using modified oak waste residues: Process optimization using response surface methodology. Journal of Industrial and Engineering Chemistry, 73, 67–77. https://doi.org/10.1016/j.jiec.2018.12.011
Sawalha, H., Bader, A., Sarsour, J., et al. (2022). Removal of dye (Methylene Blue) from wastewater using bio-char derived from agricultural residues in Palestine: Performance and isotherm analysis. Processes, 10, 2039. https://doi.org/10.3390/pr10102039
Sukla Baidya, K., & Kumar, U. (2021). Adsorption of Brilliant Green dye from aqueous solution onto chemically modified areca nut husk. South African Journal of Chemical Engineering, 35, 33–43. https://doi.org/10.1016/j.sajce.2020.11.001
Suma, Y., Pasukphun, N., Eaktasang, N., & Laor, P. (2019). Preliminary study of dye removal from aqueous solution using elephant dung activated carbon. In: IOP Conference Series: Earth and Environmental Science (Vol. 291, p. 012013). Institute of Physics Publishing. https://doi.org/10.1088/1755-1315/291/1/012013
Sun, Z., Qu, K., Cheng, Y., et al. (2021). Corncob-derived activated carbon for efficiently adsorption dye in sewage. ES Food & Agroforestry. https://doi.org/10.30919/esfaf473
Thillainayagam, B. P., Nagalingam, R., & Saravanan, P. (2022). Batch and column studies on removal of Methylene Blue dye by microalgae biochar. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-03038-3
Tie, J., Zheng, Z., Li, G., et al. (2019). Removal of an anionic azo dye Direct Black 19 from water using white mustard seed (Semen sinapis) protein as a natural coagulant. Journal of Water Reuse and Desalination, 9, 442–451. https://doi.org/10.2166/wrd.2019.018
Tolkou, A. K., & Zouboulis, A. I. (2020). Application of composite pre-polymerized coagulants for the treatment of high-strength industrial wastewaters, (Switzerland). Water, 12, 1258. https://doi.org/10.3390/W12051258
Vijayaraghavan, G., & Shanthakumar, S. (2016). Performance study on algal alginate as natural coagulant for the removal of Congo Red dye. Desalination Water Treat, 57, 6384–6392. https://doi.org/10.1080/19443994.2015.1008578
Wang, Y., Zhu, L., Wang, X., et al. (2018). Synthesis of aminated calcium lignosulfonate and its adsorption properties for azo dyes. Journal of Industrial and Engineering Chemistry, 61, 321–330. https://doi.org/10.1016/j.jiec.2017.12.030
Yeow, P. K., Wong, S. W., & Hadibarata, T. (2021). Removal of azo and anthraquinone dye by plant biomass as adsorbent – A review. Biointerface Res Appl Chem, 11, 8218–8232.
Zazycki, M. A., Godinho, M., Perondi, D., et al. (2018). New biochar from pecan nutshells as an alternative adsorbent for removing Reactive Red 141 from aqueous solutions. Journal of Cleaner Production, 171, 57–65. https://doi.org/10.1016/j.jclepro.2017.10.007
Zewde, D., & Geremew, B. (2022). Removal of Congo Red using Vernonia amygdalina leaf powder: Optimization, isotherms, kinetics, and thermodynamics studies. Environmental Pollutants and Bioavailability, 34, 88–101. https://doi.org/10.1080/26395940.2022.2051751
Zhang, W. X., Lai, L., Mei, P., et al. (2018). Enhanced removal efficiency of acid red 18 from aqueous solution using wheat bran modified by multiple quaternary ammonium salts. Chemical Physics Letters, 710, 193–201. https://doi.org/10.1016/j.cplett.2018.09.009
Zhang, P., O’Connor, D., Wang, Y., et al. (2020). A green biochar/iron oxide composite for methylene blue removal. Journal of Hazardous Materials, 384, 121286. https://doi.org/10.1016/j.jhazmat.2019.121286
Zhao, Z., Yang, Y., Xu, L., et al. (2022). Amino acid-doped polyaniline nanotubes as efficient adsorbent for wastewater treatment. Journal Chemistry, 2022, 2041512. https://doi.org/10.1155/2022/2041512
Zhao, F., Mu, B., Zhang, T., et al. (2023). Synthesis of biochar/clay mineral nanocomposites using oil shale semi-coke waste for removal of organic pollutants. Biochar, 5, 1–21. https://doi.org/10.1007/s42773-023-00205-1
Acknowledgements
We acknowledge support of this work by the project “Advanced Nanostructured Materials for Sustainable Growth: Green Energy Production/Storage, Energy Saving and Environmental Remediation” (TAEDR-0535821) which is implemented under the action “Flagship actions in interdisciplinary scientific fields with a special focus on the productive fabric” (ID 16618), Greece 2.0 – National Recovery and Resilience Fund and funded by European Union NextGenerationEU.
Funding
Open access funding provided by HEAL-Link Greece.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Eleftheria K. Tsoutsa, Athanasia K. Tolkou, George Z. Kyzas, and Ioannis A. Katsoyiannis. The first draft of the manuscript was written by Eleftheria K. Tsoutsa and Athanasia K. Tolkou and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
We undertake and agree that the manuscript submitted to your journal has not been published elsewhere and has not been simultaneously submitted to other journals.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsoutsa, E.K., Tolkou, A.K., Kyzas, G.Z. et al. An Update on Agricultural Wastes Used as Natural Adsorbents or Coagulants in Single or Combined Systems for the Removal of Dyes from Wastewater. Water Air Soil Pollut 235, 178 (2024). https://doi.org/10.1007/s11270-024-06979-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-06979-9