Abstract
In the Mediterranean region, numerous wetlands co-exist with competitive agricultural systems. Although wetlands’ hydrology (in water volume, source, and residence time terms) plays a major factor in their ecosystem structure, many of these wetlands have been subjected to different degradation processes. One such case is the Tablas de Daimiel National Park (TDNP), which witnesses one of its most environmentally sensitive times. In this context, scarce information is available on the sulfur (S) contents in this wetland. To diagnose this wetland’s degradation status, 43 soil samples were selected and analyzed to investigate S contents in the surface soils (0 to 30 cm) of the inundated floodplain area of the TDNP. They represent different degrees of exposure to pollutants and exposure to flooding. Sulfur determination was carried out by X-ray fluorescence. The S contents in the area range between 1.85 and 37.15 g kg−1, with a mean value of 13.71 g kg−1, which implies marked variability throughout this wetland. The highest values were observed near the wetland entrance via the Gigüela River, while the lowest ones appeared at its exit, with a relatively uniform gradient from the entrance to the exit. The relatively high S concentrations observed at sites other than the wetland entrance can be probably related to the continuous non-point source contamination with P, N, and S fertilizer applications, via runoff agricultural production systems. The results of this study are essential for understanding and predicting total S variability within the TDNP (Spain), which can interfere with soil management practices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The primary source of soil nutrients is the weathering of minerals in natural settings. Today, however, many human activities are also significant factors that influence soil nutrients. Soil nutrient levels vary dynamically with land cover and land management approaches, especially abrupt land-use/land cover changes (Gross & Harrison, 2019). Many of the anthropogenic actions that are implemented in agriculture (to improve soil nutrient status for growing food crops) or in wastewater treatment etc. can lead to soil and environment contamination. In some regions, intensive livestock production (e.g., pigs) and the application of large quantities of manure and synthetic fertilizers to croplands have led to environmental problems (Cameira et al., 2021).
Sulfur is an important element of both natural and anthropogenic origins (Landers et al., 1983). Together with N, P, K, Ca, and Mg, sulfur is one of the essential macro-elements required for normal plant growth (Grant et al., 2012). Unlike N and P, very few studies are available on S cycling in nature to know if S intervenes with the structure, function, and productivity of wetland ecosystems, and how it controls the physiological and biochemical functions of wetland plants.
Soils participate and regulate nutrient cycles in ecosystems, and soil nutrients are one of the most important soil characteristics (Sterner & Elser, 2017). Despite the importance of S for plant growth being acknowledged, this element has received little attention about its cycling in soils. This is probably because fertilizers and atmospheric inputs supply soils with adequate amounts of S; therefore, the problems encountered are mainly concerned with contamination of the environment with excessive S rather than S deficiency (Piotrowska-Długosz et al., 2017). So, there is a need to distinguish between the contributions of different sources to evaluate the influence of environmental factors and anthropogenic practices (Lucassen et al., 2006; Sun et al., 2003).
Sulfur is ubiquitous in the environment (Hermes et al., 2022), with many natural sources in the soil including organic matter (OM) and primary minerals (e.g., gypsum and pyrite) which release plant-available S through mineralization and weathering, respectively (Mitchell et al., 2011; Zak et al., 2021). In addition, atmospheric deposition of sulfur oxides (from fossil fuel combustion; Klimont et al., 2013) that arise primarily from anthropogenic activities and leachates of agricultural soils loaded with fertilizers and fungicides and the increasing application of wastewater and sewage sludge to the soil are significant sources of soil S. Prediction of the influence of environmental conditions on S content in wetland ecosystems requires precise quantification of S in these soils. Wetlands exposed to excessive S deposition can potentially store significant amounts of S. Keeping in mind that these wetlands can accumulate also heavy metals (HMs) (Rinklebe et al., 2007, Reddy & Delaune, 2008, Prokisch et al., 2009, Jimenez-Ballesta et al., 2016), these ecosystems can be considered true sink filters that may retain HMs and S as well. Although it is well-established that atmospheric deposition acts as a major source of S to agricultural soils (Hinckley et al., 2020), some authors (Sanchez, 2019) claimed that S deficiency is increasing worldwide, particularly in the tropics. This widespread occurrence of S deficiency can be attributed to the increasing use of NPK fertilizers that contain no S and also to the breeding of high-yielding crop cultivars that require more S, along with reduced deposition of atmospheric S downwind from industrial urban centers as an air pollution control regime.
Whatever the origin of soil S, either because of intensive application of fertilizers and sulfur pesticides to croplands or because of atmospheric deposition (what is known as “acid rain”), environmental implications certainly occur. Researchers have done a lot of work on the key processes of sulfur biogeochemical cycling, but focused for example on the spatial patterns of sulfur forms (Chamber et al., 2006) or on the distribution and accumulation of sulfur in soil-vegetation system (Chen & Sun, 2020; Lu et al., 2020).
Thus, little attention has been paid to S occurrence in the Tablas de Daimiel National Park (TDNP), despite the combined influence that the natural factors and human activities can play in this respect. This ecosystem is a genuine Mediterranean wetland in the Iberian Peninsula. Specifically, the effect of climate change, the growing human population, and intensive agricultural activities have led to a drastic decrease in the wetland area that was reduced from 3030 ha in 2014 to only 50 ha in 2022. In fact, this 50-ha wetland is currently subjected to drought and salinization processes, which lead to deterioration of the environmental functions of this wetland ecosystem, including regulating climate, controlling soil erosion and water quality, and its contribution as a recreational environment.
It was declared a Nature Park, or Protected Nature Reserve, in 1973. In 1980, the national park was extended and UNESCO included Las Tablas in a biosphere reserve. Since 1982, it has been included in the List of Wetlands of International Importance (according to the Ramsar Convention). The importance of this wetland is very relevant since it receives fresh and brackish flood water inflows and supports a rich aquatic vegetation of emergent and submergent species. In addition, this wetland supports the hydrological cycle, regulates climate change, and provides many ecosystem services to biodiversity, while supporting several economic services. In this way, Las Tablas wetland performs critical ecosystem functions and services as stopovers for migratory birds, critical nursing grounds, production of raw materials, land protection, erosion control, and especially carbon sequestration.
The complex hydrological conditions of the TDNP (Aguilera et al., 2013), together with periodic alternations of anaerobic/aerobic states, changes in groundwater level, fluctuation on soil salinity caused by water entering from the Gigüela River, chemicals discharged from the surrounding treatment plants, and the prolonged use of fungicides and fertilizers containing S and Cu (e.g., Cu sulfate, Cu oxychloride), all make the biochemical S cycle in this wetland extremely complex.
Wetland ecosystems are part of our natural wealth and are important features in the landscape that provide numerous beneficial services for people and for wildlife. Policymakers recognize the importance of healthy wetlands for achieving the Sustainable Development Goals (SDGs) and are concerned about their degradation. In this way, taking into account the fact that S cycling controls the release of HMs at the soil–water interface (Gao et al., 2016; Huo et al., 2015), it is necessary to identify the pattern of soil S content in this unique park, especially if the wetland receives effluent from the surrounding urban sewage treatment plants, which is the case in the present study. In this context, more research should be conducted to determine S content in relation to this wetland degradation status. We hypothesize that additional anthropogenic processes may modify the wetland S fingerprint. Therefore, our main objectives are to (1) determine the total S content in the inundated flooded zone of the TDNP; (2) investigating how environmental factors affect S content; and (3) assess possible S contamination levels.
2 Material and Methods
2.1 Study Area
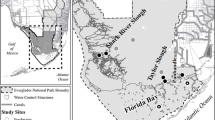
The TDNP is located in the NW zone of the natural region of La Mancha, more specifically in the western third of the geological domain of the Manchega Plain (Fig. 1). It is developed in a semi-arid climate by concentrating both surface water and groundwater from a major part of the Upper Guadiana Basin.
Location map of the TDNP wetland and the 43 sampling points chosen to monitor S content. Samples 1, 21, 37, 38, 39, 41, and 42 (located outside the flood zone) represent points of zones with potentially contaminating sources, while sample 43 denotes the wetland’s exit area. Its coordinates are as follows: DMS coordinates 39° 08′ 60.00″ N − 3°39′ 59.99″ W; UTM coordinates 30S 442394.21353951 4,333,633.9867097
The TDNP was declared as such in 1973 and covers an area of 1874 ha, which was extended, in 2014, to the current 3030 ha. It has been markedly altered in relation to its natural conditions, and groundwater use for agricultural purposes has been intense, which has caused its average piezometric level to drop 30 m during the 1989–1996 period (Mejías, 2012).
The wetland is formed by the confluence of two rivers: the Guadiana and the Gigüela. The former is inextricably linked with the groundwater of Aquifer System 23 (Mejías, 2014), currently groundwater body Mancha Occidental I, Mancha Occidental II, and Rus-Valdelobos, which drain into Los Ojos del Guadiana (“The Eyes of the Guadiana”). This has resulted in the birth of the river bearing the same name and creating, together with the Gigüela River, a unique setting where the relation between surface and groundwater plays a decisive role in the existence, viability, and environmental evolution of the protected area. The TDNP came to life and has been marked by the imprint of human presence, especially since the second half of the twentieth century, which triggered the defense of a number of occasionally opposing and hardly reconcilable interests between the preservation of environmental values and the region’s socio-economic development.
From a geological point of view, the basement consists of Paleozoic quartzites and slates. The Neogene series rests discordantly on the basement, which consists of detritus layers at the base and carbonates at the top, which constitute the upper regional aquifer. In hydrological terms, the TDNP occurs in the upper basin of the Guadiana River in the Groundwater Body of Mancha Occidental I (Fig. 1). The relief is gentle, with an altitude ranging between 600 and 750 m a.s.l. The fluvial network is critical to this singular natural setting, whose wetland is generated at the confluence of the Gigüela and Guadiana Rivers.
So these surface and groundwater discharges result in the flooding of this zone, which are known as fluvial wetlands whose water has a different chemical composition: sulfate-rich water from the Gigüela River and carbonate-rich water from the Guadiana River (Garcia Hidalgo et al., 1995). This distinctive trait in the quality of confluent waters confers the system a set of singularities and environmental values that make it an ecosystem that is practically unique in Europe.
Since the mid-twentieth century, the combined effects of desiccation works and intensive groundwater use for agricultural purposes have led to a markedly modified situation in which the disappearance of the Los Ojos del Guadiana in the summer of 1983 was detected. This situation persisted until January 2012 when, as a result of the climatologic wet sequence over 2009/2013, some Guadiana springs began to flow again. This produced surface runoff allowing groundwater contributions to the river to reach an estimated 11 Mm3 in 2014, which was 83% lower than the average water discharge drained under natural conditions (Mejías, 2019).
Typically, plant diversity in the groundwater-fed wetlands is extraordinarily high (Bedford & Godwin, 2003). This is the case of the TDNP where the vegetation is dominated by reed (Phragmites australis), followed by saw-sedge (Cladium mariscus) and cattail (Typha domingensis); this is in addition to other unique species such as buttercup (Ranunculus trichophyllus), locally known as “Manzanilla,” Veronica anagallis-aquatica, limonium (Limonium dichotomum, L. costae. L. longibracteatum), Juncus maritimus, Tamarisk (Tamarix gallica, T. canariensis), calamino (Salsola vermiculata), and Loosestrife (Lythrum salicaria) (Cirujano et al., 1996; Alvarez-Cobelas et al., 2001).
2.2 Soil Sample Collection
Generally, the wide variability in S concentrations in a field requires representative sampling (Bloem et al., 2001). According to this criterion, samples were taken in the upper 30 cm of soil in a series of transects designed along the wetland, mainly in the NE-SW direction (Fig. 1). These sampling sites were selected using a geographic information system (GIS). All the sampled sites were located using a handheld global positioning system (GPS) device. Figure 2 depicts the details of the sampling process.
Extraneous objects such as plant foliage and roots as well as rocks and shells were removed from soil samples before placing them in plastic bags. Soil samples were taken back to the laboratory to be air-dried.
2.3 Chemical Analysis
Sulfur was determined by X-ray fluorescence (XRF) spectroscopy (Kalnicky & Singhvi, 2001; Weindorf et al., 2012 and 2014), using a Philips PW 2404 spectrophotometer model. Samples were ground in an agate mortar and then pearls with lithium borate were formed. Quality control was achieved by a duplicate analysis of certified soil reference materials. Calcium carbonate was assayed by the calcimeter Bernard method and organic matter (OM) was determined by the loss-on-ignition method (dry soils were combusted at 550 °C in a muffle furnace for 4 h). Electrical conductivity (EC) was measured using an EC meter in a 1:5 soil to water extract.
Once samples had arrived at the laboratory, they were decanted to obtain saturation extracts. Sulfate (SO42−) concentrations were also determined in this extract by ion chromatography (Metrohm 792 Basic IC).
2.4 Environmental Evaluation
Although a wide range of methods are followed today to evaluate the degree of soil contamination, the method proposed by Muller (1969), namely the geoaccumulation index (Igeo), is one of the most widely used methods; therefore, it was adopted in the present work:
where Igeo is the geoaccumulation index; Cs is the measured S concentration in soil; and Bn is the background S level. Factor 1.5 was used to correct possible variations in the background values. The regional geochemical background was determined in Castilla-La Mancha soils, but they did not include S. Therefore, the Bn (also namely reference value, RV, in the bibliography), defined as the 75th percentile, has been used after excluding outliers. This Bn method of determination has been applied by Alfaro et al. (2015) and Bocardi et al. (2020). Based on the magnitude of Igeo, the contamination status of the soil samples was evaluated as non-contaminated (class 0) with Igeo < 0, slightly contaminated (class 1) with 0 < Igeo < 1, mildly contaminated (class 2) with 1 < Igeo < 2, moderately contaminated (class 3) with 2 < Igeo < 3, heavily contaminated (class 4) with 3 < Igeo < 4, highly contaminated (class 5) with 4 < Igeo < 5, and extremely contaminated (class 6) with Igeo > 5.
2.5 Statistical Analysis
We used the 19.0 for Windows version, SPSS Inc., IL, USA) with an institutional license for the University of Castilla-La Mancha (Spain). Correlation analysis was performed to calculate the Pearson’s correlation coefficients between the soil contents of S and each of carbonate, EC, organic matter, and SO42−.
3 Results and Discussion
All the soils employed in the investigated wetland were calcareous (mean of 31.6%, st dev. of 6.5, a minimum of 2.2%, and a maximum of 72.4%; Table 1). Table 1 also includes the data about OM contents (range of 3.6–78.6% with a mean of 25.07%), EC (range of 0.29–7.64 dS m−1 with a mean of 2.06 dS m−1), and sulfates in the saturation extract (range of 1.59–15,939.5 mg·l−1 with a mean of 2407.7 mg·l−1).
Table 1 shows the descriptive statistics of S in the TDNP (and their immediate environment) and some physico-chemical properties. The variations in soil S content are shown in Fig. 3. The S contents fall within the range from 1.85 to 37.15 g kg−1, with a mean of 13.71 g kg−1. This reveals marked variability throughout the wetland. A relatively high degree of variability of soil S content was indicated by the coefficient of variation (CV), which was 21%. The highest values were for the samples located near the entrance to the TDNP via the Gigüela River, while the lowest were detected at its exit. They followed a relatively uniform trend from one point to another. The explanation for this trend has to do with the usual entry of brackish water (rich in sulfate) through the Gigüela River. These value patterns in soil strikingly differ from those reported in the literature (Devai et al., 1984, 1996).
Studies that have investigated generic reference S levels in Spain are rare, nor are they common in other parts of the world. We carried out a literature review, which we present in Table 2. The distribution of the inorganic and organic S forms has been reported for salt marsh soils of California (Kaplan et al., 1963) and Maryland (Haering et al., 1989) and also for Florida marine-freshwater peats (Casagrande et al., 1977).
When comparing the results obtained for S in this study and the levels according to the cited authors, marked accumulation was evidenced in the TDNP. Several reasons can explain such differences. The sampled horizons (0–30 cm) were selected because they provided more information on contamination levels due to anthropogenic sources. In this way, and based on our results, the sulfur coming mainly from the river flows into the wetland (Gigüela River) which agrees with what was mentioned by Garcia Hidalgo et al., (1995): the Gigüela River carries brackish water. But also maybe there is another route that anthropogenic S is being delivered since relatively high values are found outside this fluvial junction (case, for example, of samples 27, 42, and 43, that in the field presented anomalous spots). Therefore, it is possible that human activities have speeded up the transportation of S from nearby areas. The risks associated with this contamination can include urban, agricultural, industrial, and, to a lesser extent, atmospheric origins.
Depending on watershed characteristics and hydraulic, nutrient, and sediment loading, wetlands accrete new soils, which differ from the physico-biological characteristics of underlying native soils (Bhomia et al., 2015; Reddy & DeLaune, 2008). The soils on the wetland floodplain, therefore, differ from those outside the wetland. For example, the drainage canals that lead to the TDNP wetland (Fig. 2a) carry wastewater, which also happens in other places of the world (Berzas et al., 2000; Karakoç et al., 2003; Mansour & Sidky, 2003).
On the one hand, S is present in all soils and derives from both parent rock materials (e.g., sulfides and sulfates, which are common minerals in the Earth’s crust) and the atmosphere (e.g., through S deposition on soil from marine aerosols, industrial gases, or gases/particulates from volcanic eruptions). As primary soil minerals, gypsum (CaSO4·2H2O) and pyrite (FeS2) should be noted. Common sulfur fertilizers are provided in Table 3.
Sulfur-bearing fertilizers actually contain more S than N or P (e.g., ammonium sulfate contains 21% N and 24% S; single superphosphate contains 9% P and 12% S) and, therefore, can be used to manage (to correct) S deficiency in plants (Weil & Mughogho, 2000).
A wetland’s landscape setting constrains the possible range of S supply and subsequent plant responses (Godwin et al., 2002). Wetlands like the TDNP are often colonized by lush vegetation which plays a significant role in OM accumulation. Therefore, wetland soils tend to accumulate OM as detritus that derives from the biota, which slowly decomposes under flooding conditions (Reddy et al., 2021). At these points, a high sulfur content can co-exist with high OM, especially because soil wetting and drying cycles control the S oxidation–reduction balance during irrigation events or wet seasons. This could be the case with greater mineralization during periods when water is lacking, especially if we bear in mind that soil temperature is an important factor that affects S mineralization.
Wetland soils are the primary component of the global biogeochemical S cycle. Sulfur has the capacity to impact the structure and productivity of wetland ecosystems; it also regulates and controls some functions of wetland plants (Wang et al., 2006). So, it is a widely recognized crucial element that is directly related to water body’s taste and odor issues (Zhu et al., 2017). However, this is not appreciated in the TDNP soils. In fact, the correlation between S and OM contents of the soil (r = 0.22) does not suggest this possibility, and this result does not agree with those obtained by Sanchez (2019) in temperate regions.
Historically, both wet and dry atmospheric S depositions have been considered the primary sources of S in remote ecosystems (Lovett, 1994). However, this is not the case in the present study because of the scarce industrial activity in the study area, which means low potential sources of S emissions. With regard to human population growth and development, Berzas et al. (2000) claimed that there is an impact of diffuse anthroponization phenomenon, motivated by land transformation to activities such as agriculture or urban growth, which occur adjacent to the protected area. However, the contribution of this factor should be cautiously interpreted.
Sulfur in wetland soils was significantly influenced by other soil properties, including soil organic matter, soil pH, and particle size (Lu et al., 2016; Tanikawa et al., 2014). Sulfate (SO42−) in the soil saturation extract ranged between 3.49 and 15,939.52 (mg·l−1) but with no detection in a few samples. De Aranda et al. (1993) provided reference values between 960 and 6724 (SO42− mg·l−1) in this same wetland’s water. Figure 4 shows correlations among S, carbonates, EC, and OM after having calculated correlations between pairs. The results are sulfur-carbonates r = − 0.19, sulfur-EC r = 0.74, sulfur-OM r = 0.22, and sulfur-sulfate anion r = 0.17. When analyzing the soils of flooding and seasonal flooding in the wetland of the Yellow River Delta of China, Lu et al. (2016) found that soil OM, salinity, and silt exerted positive loadings on S. However, the results obtained in this study of the determination coefficient (R2) between S and other soil properties were between 0.3683 and 0.0374. As is known, the higher the R2, the lower the variability. Therefore, the coefficient of determination suggests that there was no strong correlation between sulfur and the other soil properties (S-EC had the maximum R2 of 0.3683, S-SO4 had the minimum R2 of 0.0374, and the remaining had R2 values of lower than 0.06). These indicated that the results were not reliable. This fact means that if S does not correlate, especially with sulfates or EC, this means that its presence must be attributed to other anthropogenic sources. But the TDNP has complex hydrological conditions, such that it goes through periodic alternation of anaerobic/aerobic state, changing the groundwater level and soil salinity. Therefore, the biochemical cycle of sulfur in this wetland becomes particularly complex.
Scatter plot and regression line of soil S content against CaCO3 content (a), EC of saturated soil extract (b), soil organic matter content (c), and soil extract sulfate content (d). The correlations between pairs are S-carbonates r = − 0.19; S-EC r = 0.74; S-OM r = 0.22; and S-sulfate anion r = 0.17
The presence of a greater proportion of total S in soils was mainly because of its insolubility in water and non-susceptibility to leaching losses. It is a reserve source of sulfur for plants and must undergo mineralization for becoming available to plants (Srinivasarao et al., 2004). In this way, it is well known that sulfur is a vital macro-element in plant growth. The presence of adequate S supply in the soil favors plant productivity by contributing to the efficient use of other essential nutrients like N, P, and K (Amâncio et al., 2009; Messick et al., 2005). It also improves defense mechanisms against pathogen attacks (Williams et al., 2002), and it is important for maintaining crop quality (Malhi et al., 2000; Till, 2010). Therefore, S deficit has a profound impact on agricultural productivity because it inhibits plant growth and grain filling in cereals, which causes loss of productivity (Scherer, 2001). For these reasons, fertilizers are often added to soils in the region of study. However, it is often difficult for farmers to master correct fertilizer applications for crop productivity. Yet, the use of controlled-release fertilizers (CRFs) is growing, which reduces the number of fertilizer application times. Consequently, excess S application to the soil is still a standing possibility and excessive S would end up in the wetlands. Another possible source of soil S stems from applying sewage sludge, with S content of 0.6–1.5% (on dry weight basis), to the surrounding soils (Whitehead, 2000). Existing data suggest that sulfur in fertilizer and soil amendments used (both new additions and legacy sulfur in the soil) is a major source of excess sulfate entering the ecosystem (Bates et al., 2002). But the amount of total sulfur used in various soil amendments, fertilizers, and fungicides around TDNP soils is unknown.
Sources of S contamination include dispersed-source contamination from surrounding agriculture and industrial activities and point source contamination from wastewater treatment plants (Sanchez-Ramos et al., 2019). Since the 1970s, many industrialized countries, particularly those in Europe and North America, have reduced their SO2 emissions by 20–60%, and this trend is growing (Ceccotti, 1996). Indeed, for many years, soils in several regions of the world have also received S incidentally from sulfate-containing N, P, and/or K fertilizers (Whitehead, 2000). Fortunately, this trend has declined during the last few decades mainly due to the reduction in the use of two sulfate-rich fertilizers that is ammonium sulfate and superphosphate, and this seems to be the case in the TDNP wetland. However, Whitehead (2000) referred to the distribution of soil S between organic and inorganic forms that varies depending on factors like the amount of OM in soil, pH, drainage status, and mineralogical composition of the soil.
3.1 Reference Value for S and Contamination Levels
The scarcity of reference data on S in soils in Spain, and consequently in wetland soils, led us to calculate the RVs. Authors like Alfaro et al. (2015) and Bocardi et al. (2020) defined the Bn as the 75th percentile after excluding outliers. According to this criterion, we propose the Bn of 17.96 (expressed as g kg−1). Using the pollution factor Igeo to determine the contamination levels in the TDNP soils, across all samples, the mean Igeo value was − 0.974 (range − 3.864 to 0.463); therefore, most soils were included in class 0 (Igeo ≤ 0) and were therefore considered not contaminated by S. Only some isolated samples can be considered slightly contaminated.
An analysis of the literature demonstrates that SO42− contamination may have toxic effects on aquatic plants and animals, including fish, invertebrates, and amphibians, which may also pose negative implications for human health (Zak et al., 2021). Soil organic S mineralization during low water table periods can provide available S for plants. Therefore, the results of this study are essential for understanding and predicting the total S variability that can interfere with the influence of soil management practices in wetland environments. In addition, this study enhances the understanding of S sources in agrosystems to help predict and limit the potential of S contamination exposure for people and wetland ecosystems.
4 Conclusions
Due to agricultural water utilization and lower precipitation levels, the water‐covered surface area of the TDNP has gradually declined. The soil S content in the flooded floodplain area generally ranges between 1.85 and 37.15 g kg−1, with a mean value of 13.71 g kg−1. This reveals marked variability throughout the wetland. The highest values were recorded for the samples located near the park entrance via the Gigüela River, with the lowest values at its exit point, and they follow a relatively uniform trend from one point to another. The present work reveals that the S content of the soil varied widely depending on its position in the floodplain. As a first approximation, this is related to the usual entry of brackish water (rich in sulfate) via the Gigüela River; other sources are those connected with the non-point source pollution that derives from anthropogenic activities (i.e., application of fertilization and sewage sludge to the soil). The results of this study are essential for understanding and predicting the contribution of external factors that can interfere with wetland management practices. They also allow the identification of sites that can be affected by contamination processes due to current excess S addition. Therefore, they can be used to predict the impact of future global changes on this wetland ecosystem.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aguilera, H., Castaño, S., Moreno, L., Jiménez-Hernández, M. E., & De La Losa, A. (2013). Model of hydrological behaviour of the anthropized semiarid wetland of Las Tablas de Daimiel National Park (Spain) based on surface water/groundwater interactions. Hydrogeology Journal, 21, 623–641. https://doi.org/10.1007/s10040-012-0950-3
Alfaro, M. R., Montero, A., Ugarte, O. M., do Nascimento, C. W. A., de AguiarAccioly, A. M., Biondi, C. M., & da Silva, Y. J. A. B. (2015). Background concentrations and reference values for heavy metals in soils of Cuba. Environmental Monitoring and Assessment, 187(1), 1–10.
Álvarez-Cobelas, M., Cirujano, S., & Sánchez-Carrillo, S. (2001). Hydrological and botanical man-made changes in the Spanish wetland of Las Tablas de Daimiel. Biological Conservation, 97, 89–98.
Amâncio, S., Tavares, S., Fernández, J., & Sousa, C. (2009). Grapevine & sulfur: Old partners, new achievements. Capítulo 02. In A. R. Kalliopi (Ed.), Grapevine Molecular Physiology & Biotechnology (2nd ed., pp. 31–52). Springer, Greece.
Amorós, J. A., Bravo, S., García-Navarro, F. J., Pérez-de-los-Reyes, C., Chacón, J. L., Martínez, J., & Jiménez-Ballesta, R. (2015). Atlas of viticultural soils of Castilla-La Mancha. UCLM, IGea and Globalcaja.
Bates, A. L., Orem, W. H., Harvey, J. W., & Spiker, E. C. (2002). Tracing sources of sulfur in the Florida Everglades. Journal of Environmental Quality, 31, 287–299.
Bedford, B. L., & Godwin, K. S. (2003). Fens of the United States: Distribution, characteristics, and scientific connection versus legal isolation. Wetlands, 23, 608–629.
Berzas, J. J., Garcia, L. F., Rodrigues, R. C., & Martin-Alvarez, P. J. (2000). Evolución de la calidad del agua de un humedal natural gestionado: Parque Nacional Tables de Daimiel (España). Investigación Del Agua, 34, 3161–3170.
Bhomia, R. K., & Reddy, K. R. (2018). Influence of vegetation on long-term phosphorus sequestration in subtropical treatment wetlands. Journal of Environmental Quality, 47, 361–370.
Bhomia, R. K., Inglett, P. W., & Reddy, K. R. (2015). Soil and phosphorus accretion rates in sub-tropical wetlands: Everglades Stormwater Treatment Areas as a case example. Science of the Total Environment, 533, 297–306.
Bloem, E., Haneklaus, S., Sparovek, G., & Schung, E. (2001). Spatial and temporal variability of sulphate concentration in soils. Communications in Soil Science and Plant Analysis, 32(9–10), 1391–1403.
Bocardi, J. M. B., Pletsch, A. L., & Quinaia, S. P. (2020). Quality reference values for heavy metals in soils developed from basic rocks under tropical conditions. Journal of Geochemical Exploration, 217, 106591.
Bowen, H. J. M. (1979). Envorionmental of chemistry of the elements. Academc Pres.
Cameira, M. R., Rolim, J., Valente, F., Mesquita, M., Dragosits, U., & Cordovil, C. M. S. (2021). Translating the agricultural N surplus hazard into groundwater pollution risk: Implications for effectiveness of mitigation measures in nitrate vulnerable zones. Agriculture Ecosystem Environment, 306, 107204.
Casagrande, D. J., Berschinski, S. K., & C, Sutton N,. (1977). Sulfur in peat-forming systems of the Okefenokee swamp and Florida Everglades: Origins of sulfur in coal. Geochimica Et Cosmochimica Acta, 41, 161–167.
Ceccotti, S. P. (1996). Plant nutrient sulphur – A review of nutrient balance, environmental impact and fertilizers. Fertilizer Research, 43, 117–125.
Chambers, R. M., & Pederson, K. A. (2006). Variation in soil phosphorus, sulfur, and iron pools among south Florida wetlands. Hydrobiology, 569, 63–70.
Chen, B. B., & Sun, Z. (2020). Effects of nitrogen enrichment on variations of sulfur in plant-soil system of Suaeda salsa in coastal marsh of the Yellow River estuary, China. Ecological Indicators, 109, 105797. https://doi.org/10.1016/j.ecolind.2019.105797
Cirujano, S., Casado, C., Bernués, M., & Camargo, J. A. (1996). Ecological study of Las Tablas de Daimiel National Park (Ciudad Real, Central Spain): Differences in water physico-chemistry and vegetation between 1974 and 1989. Biological Conservation, 75, 211–215. https://doi.org/10.1016/0006-3207(95)00079-8
De Aranda, G., García, J., & Martín-Montalvo, J. M. (1993). Evolución de la calidad de las aguas del Parque Nacional de las Tablas de Daimiel (Ciudad Real) durante el período comprendido desde 1988 a 1993. Ecología, 7, 503–519.
Dévai, I. D., Csaba, W., Ilona, W., Dévai, G., Zoltán, D., János, H., Gyula, S., & Ferenc, M. (1984). Detection of elementary sulphur in freshwater sediments. Environmental Pollution Series b, Chemical and Physical, 8, 155–160. https://doi.org/10.1016/0143-148X(84)90025-9
Devai, I. D., Reddy, K. R., Delaune, R. D., & Graetz, D. (1996). Elemental sulfur content of wetland soils in Florida. Act. Gio. Debr. Oecol. Humg, 6, 7–12.
Gao, Z. Q., Fang, H. J., Bai, J. H., et al. (2016). Spatial and seasonal distributions of soil phosphorus in a short-term flooding wetland of the Yellow River Estuary, China. Ecological Informatics, 31, 83–90.
García Hidalgo, J. F., Temiño, J., De Bustamante, I., & Segura, M. (1995). Evolución sedimentaria reciente de las Tablas de Daimiel (Ciudad Real). Geogaceta, 8, 87–89.
Godwin, K. S., Shallenberger, J. P., Leopold, D. J., & Bedford, B. L. (2002). Linking landscape properties to local hydrogeologic gradients and plant species occurrence in minerotrophic 23 fens of New York State, USA: A Hydrogeologic Setting (HGS) framework. Wetlands, 22, 722–737.
Grant, C. A., Mahli, S. S., & Karamanos, R. E. (2012). Sulfur management for rapeseed. Field Crops Research, 128, 119–128.
Gross, C. D., & Harrison, R. B. (2019). The case for digging deeper: Soil organic carbon storage, dynamics, and controls in our changing world. Soil System, 3, 28. https://doi.org/10.3390/soilsystems3020028
Haering, K. C., Rabenhorst, M. C., & Fanning, D. S. (1989). Sulfur speciation in some Chesapeake Bay tidal marsh soils. Soil Science Society of America Journal, 53, 500–505.
Hermes, A. L., Dawson, T. D., & Hinckley, E. S. (2022). Sulfur isotopes reveal agricultural changes to the modern sulfur cycle. Environment Research Letters, 17, 054032.
Hinckley, E. L. S., Crawford, J. T., Fakhraei, H., & Driscoll, C. T. (2020). A shift in sulfur-cycle manipulation from atmospheric emissions to agricultural additions. Nature Geoscience, 13, 597–604.
Huo, S., Zhang, J., Yeager, K., Xi, B., Qin, Y., He, Z., & Wu, F. (2015). Mobility and sulfidization of heavy metals in sediments of a shallow eutrophic lake, Lake Taihu, China. Journal of Environmental Sciences, 31, 1–11. https://doi.org/10.1016/j.jes.2014.12.003
Jiménez-Ballesta, R., García-Navarro, F. J., Bravo, S., Amorós, J. A., & Perez-de los Reyes C, Mejias M,. (2016). Environmental assessment of potential toxic trace element contents in the inundated floodplain area of Tablas de Daimiel wetland (Spain). Environmental Geochemistry and Health, 39, 1159–1177.
Jiménez-Ballesta, R., Bravo, S., Amoros, J. A., Pérez-de-los-Reyes, C., García-Pradas, J., Sanchez, M., & García-Navarro, F. J. (2022). Soil and leaf mineral element contents in Mediterranean vineyards: Bioaccumulation and potential soil pollution. Water, Air, & Soil Pollu. https://doi.org/10.1007/s11270-021-05485-6
Kabata-Pendias, A. (2011). Trace elements in soils and plants; CRC Press: Boca Raton. FL.
Kalnicky, D., & Singhvi, R. (2001). Field portable XRF analysis of environmental samples. Journal of Hazardous Materials, 83, 93–122.
Kaplan, J. R., Emery, K. O., & Rittenberg, S. C. (1963). The distribution and isotopic abundance of sulphur in recent marine sediments off southern California. Geochimica Et Cosmochimica Acta, 27, 297–331.
Karakoç, G., Ünlü, E., & Katırcıoğlu, H. (2003). Water quality and impacts of pollution sources for Eymir and Mogan Lakes (Turkey). Environment Int, 29(1), 21–27.
Klimont, Z., Smith, S. J., & Cofala, J. (2013). The last decade of global anthropogenic sulfur dioxide: 2000–2011 emissions. Environmental Research Letters, 8, 014003.
Landers, D. H., David, M. B., & Mitchell, M. J. (1983). Analysis of organic and inorganic sulfur constituents in sediments, soils, and water. International Journal of Environmental Analytical Chemistry, 14, 245–256.
Li, X. H., Liu, J. S., Sun, X. J., Zhu, Z. L., Zhang, X. J. (2009). Composition and Vertical Distribution of Soil Sulfur in Wetland Soil Under Calamagrostis angustifolia in Sanjiang Plain. Journal of Ecology and Rural Environment, 25(2), 34-38. CNKI:SUN:NCST.0.2009-02-008
Lovett, G. M. (1994). Atmospheric deposition of nutrients and pollutants in North America: An ecological perspective. Ecological Applications, 4, 629–650.
Lu, Q., Bai, J., Fang, H., Wang, J., Zhao, Q., & Jia, J. (2016). Spatial and seasonal distributions of soil sulfur in two marsh wetlands with different flooding frequencies of the Yellow River Delta, China}. Ecological Engineering, 96, 63–71. https://doi.org/10.1016/j.ecoleng.2015.10.033
Lu, Q., Bai, J., Yan, D., Cui, B., & Wu, J. (2020). Sulfur forms in wetland soils with different flooding periods before and after flow-sediment regulation in the Yellow River Delta, China. Journal of Cleaner Production, 276, 122969. https://doi.org/10.1016/j.jclepro.2020.122969
Lucassen, E. C., Smolders, A. J. P., Boedeltje, G., Munckhof, V., & Roelofs, J. (2006). Groundwater input affecting plant distribution by controlling ammonium and iron availability. Journal of Vegetation Science, 17(4), 425–434. https://doi.org/10.1658/1100-9233(2006)17[425:GIAPDB]2.0.CO;2Y
Malhi, S. S., Heier, K., & Solberg, E. (2000). Effectiveness of elemental S fertilizers on forage grass. Canadian Journal of Soil Science., 80, 105–112.
Mansour, S. A., & Sidky, M. S. M. (2003). Ecotoxicological studies. 6. The first comparative study between Lake Qarun and Wadi El-Rayan wetland (Egypt), with respect to contamination of their major components. Food Chemistry, 82, 181–189.
Mejías, M. (2019). Las Tablas de Daimiel: A singular space in the National Parks Network. Boletín Geológico y Minero, 130(4), 691–710. https://doi.org/10.21701/bolgeomin.130.4.006
Mejías, M., (Ed.). (2014). Las Tablas y los Ojos del Guadiana: Agua, paisaje y gente; Instituto Geológico y Minero de España, Organismo Autónomo Parques Nacionales: Madrid, Spain, pp. 360.
Mejías, M., López, J., & Martínez, L. (2012). Características hidrogeológicas y evolución piezométrica de la Mancha Occidental. Influencia del periodo húmedo 2009–2011. Boletín Geológico y Minero, 123(2), 91–108.
Messick, D. L., Fan, M. X., & Brey, C. (2005). Global sulfur requirement and sulfur fertilizer. Special number. 283. In L. De Kok y, & E. Schnug (Eds.), Proceedings of the first Sino-German workshop on aspect of sulfur nutrition of plants (pp. 97–104). Shenyang, China.
Mitchell, M. J., et al. (2011). Comparisons of watershed sulfur budgets in southeast Canada and northeast US: New approaches and implications. Biogeochem, 103, 181–207.
Müller, G. (1969). Index of geo-accumulation in sediments of the Rhine River. Geo Journal, 2, 108–118.
Piotrowska-Długosz, A., Anetta, S. Z., Jacek, D., & Dariusz, G. (2017). Spatio-temporal variability of soil sulfur content and arylsulfatase activity at a conventionally managed arable field. Geoderma, 295, 107–118.
Prokisch, J., Szeles, E., Kovacs, B., Gyori, Z., Nemeth, T., & West, L. (2009). Sampling strategies for testing and evaluation of soil contamination in Riparian Systems at the Tisza River Basin, Hungary. Communications in Soil Science and Plant Analysis, 40, 391–406.
Reddy, K. R., & DeLaune, R. D. (2008). Biogeochemistry of wetlands: Science and applications (1st ed.). CRC Press.
Reddy, K. R., Hu, J., Villapando, O., Bhomia, R. K., Vardanyan, L., & Osborne, T. (2021). Long-term accumulation of macro- and secondary elements in subtropical treatment wetlands. Ecosphere, 12(11), e03787. https://doi.org/10.1002/ecs2.3787
Rinklebe, J., Franke, C., & Neue, H. U. (2007). Aggregation of floodplain soils as an instrument for predicting concentrations of nutrients and pollutants. Geoderma, 141, 210–223.
Sanchez, P. A. (2019). Properties and management of soils in the tropics, Chapter 15 – Sulfur. Cambridge University Press
Scherer, H. W. (2001). Sulphur in crop production – Invited paper. European Journal of Agronomy, 14, 81–111.
Skyllberg, U., Persson, A., Tjerngren, I., Kronberg, R. M., Drott, A., Meili, M., & Björn, E. (2021). Chemical speciation of mercury, sulfur and iron in a dystrophic boreal lake sediment, as controlled by the formation of mackinawite and framboidal pyrite. Geochimica Et Cosmochimica Acta, 294, 106–125. https://doi.org/10.1016/j.gca.2020.11.022
Sparks, D. L. (2003). Environmental soil chemistry (2nd ed., p. 352). Elsevier.
Srinivasarao, C., Ganeshamurthy, A. N., Ali, M., Singh, R. N., & Singh, K. K. (2004). Sulfur fractions, distribution, and their relationships with soil properties in different soil types of major pulse-growing regions of India. Communications in Soil Science and Plant Analysis, 35, 2757–2769.
Sterner, R. W., & Elser, G. (2017). Ecological stoichiometry. Princeton University Press.
Sun, B., Zhou, S., & Zhao, Q. (2003). Evaluation of spatial and temporal changes of soil quality based on geostatistical analysis in the hill region of subtropical China. Geoderma, 115, 85–99.
Suri, D., Sharma, V. K., Kumar, P., Upadhayay, R. G., & Nazir, G. (2021). Sulphur dynamics under different land uses of Outer Himalayan region of Himachal Pradesh. Environment Conservation Journal, 22(3), 265–270.
Syers, J. K., Skinner, R. J., & Curtin, D. (1987). Soil and fertilizer sulphur in UK agriculture. Proceedings of the Fertilizer Society, 264, 43.
Tanikawa, T., Noguchi, K., Nakanishi, K., Shigenaga, H., & Nagakura, J. (2014). Sequential transformation rates of soil organic sulfur fractions in two-step mineralization process. Biology and Fertility of Soils, 2014(50), 225–237.
Till, A. R. (2010). Sulphur and sustainable agriculture. Intenational Fertilizer Industry Association (1st Ed.). Paris, France.
Wang, J., Solomon, D., Lehmann, J., Zhang, X., & Amelung, W. (2006). Soil organic sulfur forms and dynamics in the Great Plains of North America as influenced by long-term cultivation and climate. Geoderma, 133, 160–172. https://doi.org/10.1016/j.geoderma.2005.07.003
Watson, C. J., & Stevens, R. J. (1986). The sulphur content of slurries and fertilizers. In Record of agricultural research, Northern Ireland, HMSO, Belfast, pp. 5–7.
Weil, R. R., & Mughogho, S. K. (2000). Sulfur nutrition of maize in four regions of Malawi. Agronomy Journal, 92, 649–656.
Weindorf, D. C., & Bakr, N. (2014). Zhu Y (2014) Advances in portable X-ray fluorescence (PXRF) for environmental, pedological, and agronomic applications. Advances in Agronomy, 128, 1–45.
Weindorf, D. C., Zhu, Y., Chakraborty, S., Bakr, N., & Huang, B. (2012). Use of portable X-ray fluorescence spectrometry for environmental quality assessment of peri-urban agriculture. Environmental Monitoring and Assessment, 184, 217–227.
Whitehead, D. C. (2000). Nutrient elements in grassland: Soil-plant-animal relationships. CABI Publishing.
Williams, J. S., Hall, S. A., Hawkesford, M. J., Beale, M. H., & Cooper, R. M. (2002). Elemental sulfur and thiol accumulation in tomato and defense against a fungal vascular pathogen. Plant Physiology., 128, 150–159.
Zak, D., Huffer, M., Heads, A., Jurasinski, G., Audet, J., Kleeberg, A., McInnes, R., Kristiansen, S., Petersen, R., Liu, H., & Goldhammer, T. (2021). Sulfate in freshwater ecosystems: A review of sources, biogeochemical cycles, ecotoxicological effects and bioremediation. Earth Science Reviews, 212, 103446. https://doi.org/10.1016/j.earscirev.2020.103446
Zhu, J., He, Y., Zhu, Y., Huang, M., & Zhang, Y. (2017). Biogeochemical sulfur cycling coupling with dissimilatory nitrate reduction processes in freshwater sediments. Environmental Reviews, 26, 121–132. https://doi.org/10.1139/er-2017-0047
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research was financially supported by the Organismo Autonomo Parques Naturales (Natural Park Autonomous Organisation) of Spain.
Author information
Authors and Affiliations
Contributions
R. Jiménez-Ballesta: conceived and designed this study, participated in collecting soil samples and writing—original draft preparation.
M. Mejias: participated in collecting soil samples and writing—original draft preparation.
S. Bravo: participated in collecting soil samples, software and formal analysis, and writing—original draft preparation.
C. Pérez-de-los-Reyes: participated in collecting soil samples and software.
J.A. Amorós: participated in collecting soil samples and software.
F.J. García-Navarro: conceived and designed this study, participated in collecting soil samples and writing—original draft preparation.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiménez-Ballesta, R., Mejias, M., Bravo, S. et al. Sulfur Signature of a Mediterranean Wetland: The Case of Soils of the Inundated Floodplain Area at the Tablas de Daimiel National Park. Water Air Soil Pollut 235, 150 (2024). https://doi.org/10.1007/s11270-024-06941-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-06941-9